Abstract
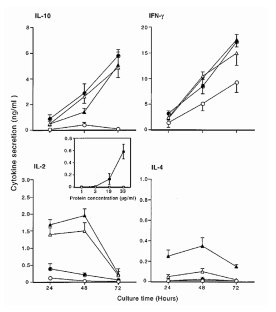
The B7 family members B7-1 and B7-2 interact with CD28 and constitute an essential T-cell co-stimulatory pathway in the initiation of antigen-specific humoral and cell-mediated immune response. Here, we describe a third member of the B7 family, called B7-H1 that does not bind CD28, cytotoxic T-lymphocyte A4 or ICOS (inducible co-stimulator). Ligation of B7-H1 co-stimulated T-cell responses to polyclonal stimuli and allogeneic antigens, and preferentially stimulated the production of interleukin-10. Interleukin-2, although produced in small amounts, was required for the effect of B7-H1 co-stimulation. Our studies thus define a previously unknown co-stimulatory molecule that may be involved in the negative regulation of cell-mediated immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscription info for Japanese customers
We have a dedicated website for our Japanese customers. Please go to natureasia.com to subscribe to this journal.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chambers, C.A. & Allison, J.P. Co-stimulation in T cell responses. Curr. Opin. Immunol. 9, 369– 404 (1997).
Lenschow, D.J., Walunas, T.L. & Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Chen, L., Linsley, P.S. & Hellstrom, K.E. Costimulation of T cells for tumor immunity. Immunol. Today 14, 483–486 (1993).
Boise, L.H., Noel, P.J. & Thompson, C.B. CD28 and apoptosis. Curr. Opin. Immunol. 7, 620–625 (1995).
Krummel, M.F. & Allison, J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183, 2533– 2540 (1996).
Walunas, T.L., Bakker, C.Y. & Bluestone, J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183, 2541– 2550 (1996).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
Moore, K.W. et al. Interleukin-10. Annu. Rev. Immunol. 11, 165–190 (1993).
Freeman, G.J. et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science 262, 909–911 (1993).
Azuma, M. et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366, 76–79 (1993).
Peach, R.J. et al. Both extracellular immunoglobulin-like domains of CD80 contain residues critical for binding T cell surface receptor CTLA-4 and CD28. J. Biol. Chem. 270, 21181–21187 (1995).
Fargeas, C.A. et al. Identification of residues in the V domain of CD80 (B7-1) implicated in functional interactions with CD28 and CTLA-4. J. Exp. Med. 182, 667–675 (1995).
Bajorath, J., Peach, R. J. & Linsley, P. S. Immunoglobulin fold characteristics of B7-1 (CD80) and B7-2 (CD86). Protein Sci. 3, 2148– 2150 (1994).
Linsley, P.S. et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1, 793–801 (1994).
Inaba, K. et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro . J. Exp. Med. 180, 1849– 1860 (1994).
Freeman, G. J. et al. B7-1 and B7-2 do not deliver identical costimulatory signals since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity 2, 523– 532 (1995).
Nakajima, A. et al. Requirement of CD28-CD86 co-stimulation in the interaction between antigen-primed T helper type 2 and B cells. Int. Immunol. 9, 637–644 (1997).
Schwartz, R. H. T cell clonal anergy. Curr. Opin. Immunol. 9, 351–357 (1997).
Georgescu, L, Vakkalanka, R.K, Elkon, K.B. & Crow, M.K. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J. Clin. Invest. 100, 2622 –2633 (1997).
Goerdt, S. & Orfanos, C. E. Other functions, other genes: Alternative activation of antigen-presenting cells. Immunity 10, 137–142 (1999).
Li, Y., McGowan, P., Hellstrom, K.E. & Chen, L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J. Immunol. 153, 421–428 (1994).
Chen, L. et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 71, 1093–1102 (1992).
Li, Y., Hellstrom, K.E., Newby, S.A. & Chen, L. Costimulation by CD48 and B7-1 induces immunity against poorly immunogenic tumors. J. Exp. Med. 183, 639–644 (1996).
Acknowledgements
We thank J. Lau and K. Jensen for editing the manuscript. This work was supported in part by the Mayo Foundation and National Institutes of Health grant CA79915. G.Z. is supported by National Institutes of Health training grant CA09127. The accession number for the human B7-H1 sequence in the GenBank is AF177937.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, H., Zhu, G., Tamada, K. et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5, 1365–1369 (1999). https://doi.org/10.1038/70932
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/70932
This article is cited by
-
PD-L1 knockdown suppresses vasculogenic mimicry of non-small cell lung cancer by modulating ZEB1-triggered EMT
BMC Cancer (2024)
-
PD-L1 expression and its significance in advanced NSCLC: real-world experience from a tertiary care center
Journal of the Egyptian National Cancer Institute (2024)
-
Role of stromal PD-L1 expression in colorectal liver metastasis
BMC Cancer (2024)
-
Profiling phagosome proteins identifies PD-L1 as a fungal-binding receptor
Nature (2024)
-
The PD-1–PD-L1 pathway maintains an immunosuppressive environment essential for neonatal heart regeneration
Nature Cardiovascular Research (2024)