Abstract
The APC gene encodes the adenomatous polyposis coli tumour suppressor protein, germline mutation of which characterizes familial adenomatous polyposis (FAP), an autosomal intestinal cancer syndrome1. Inactivation of APC is also recognized as the key early event in the development of sporadic colorectal cancers2,3, and its loss results in constitutive activity of the
Similar content being viewed by others
Main
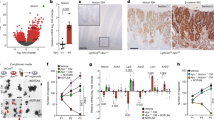
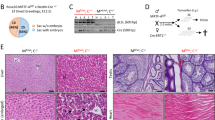
We have previously used a conditional gene deletion approach to study the immediate effects of loss of Apc on differentiation, proliferation and migration in the murine small intestine6. These studies have shown that loss of Apc leads to unrestricted proliferation within the intestinal crypt, resulting in multiple changes, including an increase in BrdU incorporation, MCM staining and dramatically enlarged crypts at four days post Apc loss6. Similar analyses of Myc deficiency have shown remarkably little effect of gene deletion on intestinal proliferation and apoptosis over a five day time course7,8. To examine how Myc affected the phenotypes associated with Apc loss, we intercrossed AhCre+Apcfl/fl mice with Mycfl/fl mice. AhCre mice carry a Cre transgene that is under control of the cytochrome p450 1A1 (CYP1A1) promoter. Mice were given three injections of
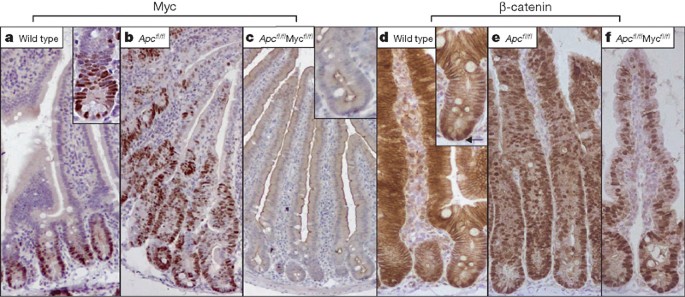
a–c, Myc shown by immunohistochemistry. a, Myc in wild-type crypts (AhCre+Apc++Myc++ mice) at four days post Cre induction. Inset shows nuclear Myc expression at the base of the crypt. b, Upregulation of Myc following Apc loss in Apc-deficient crypts (AhCre+Apcfl/flMyc+/+ mice) at four days post Cre induction. The immunohistochemistry shows nuclear Myc in every Apc-deficient cell in the crypt. c, Loss of Myc protein in double-mutant crypts (AhCre+Apcfl/flMycfl/fl) at four days following Cre induction. Inset shows that no cells express Myc within the crypt. d–f, Upregulation of
To confirm co-incident Apc and Myc loss, we performed in situ hybridization for Apc and immunohistochemistry for Myc and
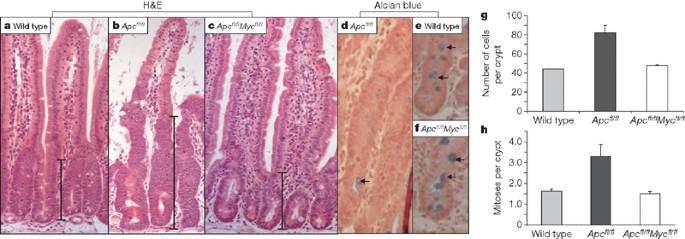
a–c, Haematoxylin and eosin (H&E)-stained sections for wild-type (AhCre+Apc+/+Myc+/+) (a), Apc-deficient (AhCre+Apcfl/flMyc+/+) (b) and double-mutant Apc- and Myc-deficient (AhCre+Apcfl/flMycfl/fl) (c) intestines. Note the enlarged crypts in the Apc-deficient mice (b) are no longer present in double-mutant intestines (c). d–f, Restored goblet cell number in double-mutant intestines. Alcian blue-stained section for Apc-deficient (AhCre+Apcfl/flMyc+/+) (d), wild-type (AhCre+Apc+/+Myc+/+) (e), and double-mutant (AhCre+Apcfl/flMycfl/fl) (f) intestines. Note the reduction in goblet cells that occurs in Apc-deficient crypts does not occur in double-mutant crypts (Mann–Whitney test, P = 0.04, n = 3). Arrows point to ‘blue’ goblet cells. g, Crypt cellularity is not increased in double- mutant crypts. Graph showing average cell number per crypt. Error bars, s.d. Unlike Apc-deficient crypts, which have a significantly increased size compared with wild type crypts (P = 0.04, Mann–Whitney test, n = 3), no difference in size was observed between wild-type and double-mutant crypts (Mann–Whitney test, P = 0.4). h, Numbers of mitotic figures per crypt are not increased in double-mutant crypts. Graph showing average number of mitoses per crypt. Error bars, s.d. Unlike Apc-deficient crypts, which have a significantly increased number of mitotic figures per crypt compared with wild-type crypts (P = 0.04, Mann–Whitney test, n = 3), no significant difference was observed between wild-type and double-mutant crypts (Mann–Whitney test, P = 0.303). In all figures n refers to the number of mice analysed. Error bars, s.d. In a–c, scale bars show size of crypt; magnification of d–f, ×600.
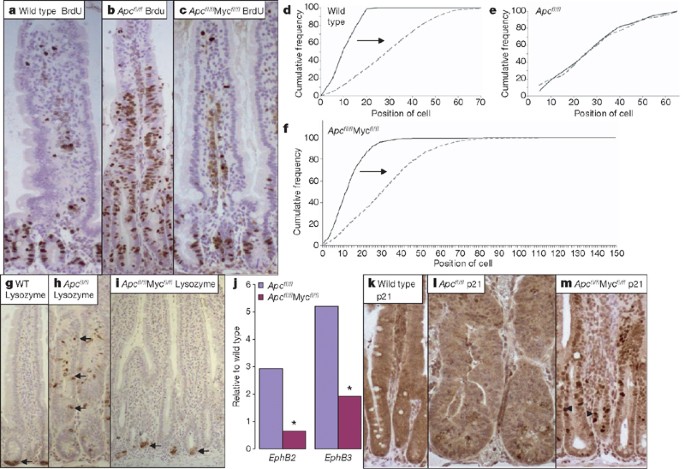
a–c, Immunohistochemistry for BrdU performed on wild-type (AhCre+Apc+/+Myc+/+) (a), Apc-deficient (AhCre+Apcfl/flMyc+/+) (b) and double-mutant (AhCre+Apcfl/fl Mycfl/fl) (c) intestines, at four days after Cre induction and two hours following BrdU injection. Note that wild-type and double-mutant crypts only incorporate BrdU within the crypt, displaying a defined proliferation zone. Apc-deficient crypts have a significantly increased number and percentage of BrdU-labelled cells (Mann–Whitney test, P = 0.04). d–f, Graphs showing the position of the BrdU-labelled cells 2 h (black solid line) and 24 h (grey dashed line) after labelling. In wild-type (AhCre+Apc+/+Myc+/+) crypts, cells migrate up the crypt–villus axis from 2 to 24 h; shown here by cells labelling at higher positions on the crypt–villus axis (arrows; Kolmogorov–Smirnov test, 2 h versus 24 h, P = 0.01). Within Apc-deficient (AhCre+Apcfl/flMyc+/+) crypts, cells label throughout the crypt–villus axis at 2 h and there is no movement 24 h later (Kolmogorov–Smirnov test, 2 h versus 24 h, P = 0.8). Within double-mutant (AhCre+Apcfl/flMycfl/fl) crypts, cells migrated up the crypt–villus axis (Kolmogorov–Smirnov test, 2 h versus 24 h, P = 0.01). g–i, Immunohistochemistry for lysozyme, performed on wild-type (WT; AhCre+Apc+/+Myc+/+) (g), Apc-deficient (AhCre+Apcfl/flMyc+/+) (h) and double-mutant (AhCre+Apcfl/flMycfl/f) (i) intestines. Positions of lysozyme-positive paneth cells are denoted by arrows. j, Real time qRT–PCR for the EphB2 and EphB3 receptor mRNAs. Note Paneth cells are no longer mislocalized in double-mutant crypts and expression of the EphB2 and EphB3 receptor mRNAs is significantly lower than in Apc-deficient crypts (*P = 0.04, Mann–Whitney test). k–m, Immunohistochemistry for p21, performed on wild-type (k), Apc-deficient (l) and double-mutant (AhCre+Apcfl/flMycfl/fl)(m) crypts. Arrowheads indicate a number of p21-positive cells in double-mutant crypts (m). Magnifications, ×400.
Levels of nuclear
Thus, we define, in an in vivo setting, a subset of Wnt target genes for which Myc is essential. This subset of target genes is critical in imposing the Apc-deficient phenotype. For example, we have previously shown that perturbation of the EphB/EphrinB system, following Apc loss, results in mislocalization of Paneth cells6,13. In the double mutants, these Tcf4 target genes were no longer transcriptionally elevated, and consistent with this, we observed normal localization of the Paneth cells in the double mutants (Fig. 3g–j). Critically, the Wnt target genes that we identify as Myc-independent are insufficient to impose the phenotypes associated with Apc deficiency.
In the double mutants, a number of genes associated with DNA and RNA replication were no longer transcriptionally upregulated (for example, DNA polymerase
Previous studies have postulated that Myc may facilitate proliferation through repression of p21 (ref. 5). This is unlikely to be a direct effect within normal intestinal crypts because there is no induction of p21 and no obvious G1 arrest when Myc is deleted7,8. However, repression of p21 by Myc was demonstrated in colorectal cancer cell lines mutant for Apc, raising the possibility that repression of p21 may only be important in the context of activated Wnt signalling. To address this, we performed immunohistochemistry for p21 (Fig. 3k–m) and found p21 to be upregulated within the crypts of double-mutant Apcfl/flMycfl/fl mice. This argues that the loss of the crypt progenitor-cell-like phenotype in double-mutant mice may be partially due to the inability to repress p21.
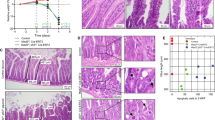
Previous reports have suggested that Myc can integrate and balance different survival signals. In certain contexts, Myc expression can lead to proliferation, whereas in other contexts, activation of Myc drives apoptosis15,16. Given the failure to see increased proliferation in the double mutants, we next investigated levels of apoptosis in the AhCre+Apcfl/flMycfl/fl intestines. Previously, we have shown that loss of Apc increases the apoptotic index in AhCre+Apcfl/fl mice. In the double-mutant AhCre+Apcfl/flMycfl/fl intestines this increase was completely blocked (wild type, 0.16% ± 0.05; Apcfl/fl, 7.3% ± 0.6; Apcfl/flMycfl/fl, 0.33 ± 0.13; Supplementary Fig. 5a), showing Myc dependency for both apoptosis and proliferation, following activation of the Wnt pathway. To investigate further the link between proliferation and death, we pulse-labelled with BrdU and followed the fate of labelled cells. Within wild-type crypts, there was a 43% increase in the number of labelled cells (as a consequence of division) between 2 and 24 h (Supplementary Fig. 5b). No such increase was observed in the AhCre+Apcfl/flMyc+/+ mice, implying that BrdU-positive cells were being deleted (Fig. 4b). This interpretation is further supported by the observation that the mitotic index was not elevated in the Cre+Apcfl/fl mice (control, 5.83 ± 0.27 s.e.; Cre+Apcfl/fl, 6.43 ± 1.77; P = 0.66, Mann–Whitney test, n = 3), despite the large increase in BrdU labelling, implying that death is occurring in either the G2 or M phases17. In accordance with this, many of the apoptotic figures observed were large—a phenomenon previously interpreted to reflect death of 4n cells at a G2/M checkpoint17. This is also consistent with reports that Myc upregulation drives cells precociously into S phase18 and
To investigate the long-term fate of doubly deficient cells, we intercrossed AhCre+Apcfl/flMycfl/fl mice with mice carrying the ROSA26 lacZR allele20 to report Cre-mediated recombination, and exposed them to dietary
We have previously shown that single deficiency of Myc leads to reduced biosynthetic activity and strong selection against Myc-deficient stem cells8. We hypothesize that, in the double mutants, surrounding wild-type stem cells outcompete the double-null cells and repopulate the crypts, much as we have seen for other alleles deleted using this strategy22,23.
In conclusion, we show that the dramatic changes conferred by Apc deficiency are entirely dependent on functional Myc. Furthermore, given that Myc is required for the persistence of Apc-deficient cells, these data show that Myc is absolutely required for the cellular and molecular changes that occur following Apc loss in the murine small intestine.
Methods
Mouse colonies
All experiments were performed under the UK Home Office guidelines. Outbred male mice from 6–12 weeks of age were used, and they were segregating for the C57BLJ and S129 genomes. The alleles used were as follows: Apc580S flox (ref. 6), Mycfl/fl(ref. 8) and AhCre (ref. 22).
Tissue isolation and analysis
To induce recombination, mice were given daily intraperitoneal injections of
References
Kinzler, K. W. et al. Identification of FAP locus genes from chromosome 5q21. Science 253, 661–665 (1991)
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996)
Korinek, V. et al. Constitutive transcriptional activation by a
β -catenin–Tcf complex in APC-/- colon carcinoma. Science 275, 1784–1787 (1997)He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998)
van de Wetering, M. et al. The
β -catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002)Sansom, O. J. et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18, 1385–1390 (2004)
Bettess, M. D. et al. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol. Cell. Biol. 25, 7868–7878 (2005)
Muncan, V. et al. Rapid loss of intestinal crypts on conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell. Biol. 26, 8418–8426 (2006)
Breitling, R., Armengaud, P., Amtmann, A. & Herzyk, P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 573, 83–92 (2004)
Tusher, V. G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. USA 98, 5116–5121 (2001)
Giles, R. H., van Es, J. H. & Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653, 1–24 (2003)
Malliri, A. et al. The Rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J. Biol. Chem. 281, 543–548 (2006)
Batlle, E. et al.
β -catenin and TCF4 mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell 111, 251–263 (2002)Oskarsson, T. & Trumpp, A. The Myc trilogy: lord of RNA polymerases. Nature Cell Biol. 7, 215–217 (2005)
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in
β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002)Green, D. R. & Evan, G. I. A matter of life and death. Cancer Cell 2002, 19–30 (2002)
Merritt, A. J., Allen, T. D., Potten, C. S. & Hickman, J. A. Apoptosis in small intestinal epithelia from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after
γ -irradiation. Oncogene 14, 2759–2766 (1997)Li, Q. & Dang, C. V. c-Myc overexpression uncouples DNA replication from mitosis. Mol. Cell. Biol. 19, 5339–5351 (1999)
Olmeda, D., Castel, S., Vilaro, S. & Cano, A.
β -catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol. Biol. Cell. 14, 2844–2860 (2003)Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999)
Sansom, O. J. et al. Cyclin D1 is not an immediate target of
β -catenin following Apc loss in the intestine. J. Biol. Chem. 280, 28463–28467 (2005)Ireland, H. et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of
β -catenin. Gastroenterology 126, 1236–1246 (2004)Hay, T., Patrick, T., Winton, D., Sansom, O. J. & Clarke, A. R. Brca2 deficiency in the murine small intestine sensitizes to p53-dependent apoptosis and leads to the spontaneous deletion of stem cells. Oncogene 24, 3842–3846 (2005)
Acknowledgements
This work was supported by the CR-UK. Thanks to D. Scarborough and Beatson technology services (T. Gilby, M. O’Prey and A. Dawson) for help with histology, M. Bishop for genotyping and K. Vousden for comments. Thanks also to Y. Hey and the PICR for microarray analysis.
Author Contribution: O.J.S., V.S.M., V.M., K.R.R., T.J.P. J.A.W., J.K.V. and D.A. conducted research for the paper. O.J.S., H.C. and A.R.C. wrote the paper. All authors discussed the results and read the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-6 with Legends, Supplementary Tables 1-4, Supplementary Methods and additional references. (PDF 1381 kb)
Rights and permissions
About this article
Cite this article
Sansom, O., Meniel, V., Muncan, V. et al. Myc deletion rescues Apc deficiency in the small intestine. Nature 446, 676–679 (2007). https://doi.org/10.1038/nature05674
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05674
This article is cited by
-
FOXO transcription factors as mediators of stress adaptation
Nature Reviews Molecular Cell Biology (2024)
-
New insights in ubiquitin-dependent Wnt receptor regulation in tumorigenesis
In Vitro Cellular & Developmental Biology - Animal (2024)
-
RUNX3 inactivates oncogenic MYC through disruption of MYC/MAX complex and subsequent recruitment of GSK3
β -FBXW7 cascadeCommunications Biology (2023)
-
mTOR inhibition suppresses Myc-driven polyposis by inducing immunogenic cell death
Oncogene (2023)
-
Metabolic profiling stratifies colorectal cancer and reveals adenosylhomocysteinase as a therapeutic target
Nature Metabolism (2023)