Abstract
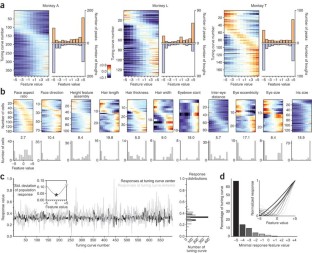
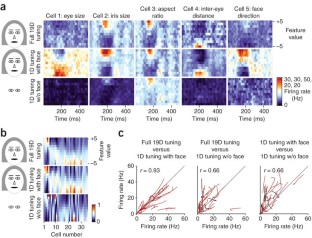
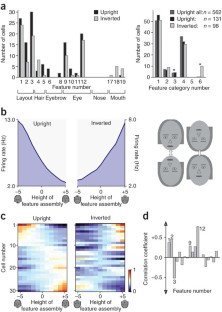
The ability of primates to effortlessly recognize faces has been attributed to the existence of specialized face areas. One such area, the macaque middle face patch, consists almost entirely of cells that are selective for faces, but the principles by which these cells analyze faces are unknown. We found that middle face patch neurons detect and differentiate faces using a strategy that is both part based and holistic. Cells detected distinct constellations of face parts. Furthermore, cells were tuned to the geometry of facial features. Tuning was most often ramp-shaped, with a one-to-one mapping of feature magnitude to firing rate. Tuning amplitude depended on the presence of a whole, upright face and features were interpreted according to their position in a whole, upright face. Thus, cells in the middle face patch encode axes of a face space specialized for whole, upright faces.
This is a preview of subscription content, access via your institution
Access options
Subscription info for Japanese customers
We have a dedicated website for our Japanese customers. Please go to natureasia.com to subscribe to this journal.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hubel, D.H. & Wiesel, T.N. Receptive fields of single neurones in the cat's striate cortex. J. Physiol. (Lond.) 148, 574–591 (1959).
Kanwisher, N., McDermott, J. & Chun, M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997).
Tsao, D.Y., Freiwald, W.A., Knutsen, T.A., Mandeville, J.B. & Tootell, R.B.H. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 6, 989–995 (2003).
Tsao, D.Y., Freiwald, W.A., Tootell, R.B.H. & Livingstone, M.S. A cortical region consisting entirely of face-selective cells. Science 311, 670–674 (2006).
Brunswik, E. & Reiter, L. Eindruckscharaktere schematisierter Gesichter. Zeitschrift Fuer Psychologie 142, 67–135 (1937).
Biederman, I. Recognition-by-components: a theory of human image understanding. Psychol. Rev. 94, 115–147 (1987).
Rhodes, G., Brennan, S. & Carey, S. Identification and ratings of caricatures: implications for mental representations of faces. Cognit. Psychol. 19, 473–497 (1987).
Benson, P.J. & Perrett, D.I. Perception and recognition of photographic quality facial carricatures: implications for the recognition of natural images. Eur. J. Cogn. Psychol. 3, 105–135 (1991).
Leopold, D.A., Bondar, I.V. & Giese, M.A. Norm-based face encoding by single neurons in monkey inferotemporal cortex. Nature 442, 572–575 (2006).
Mel, B. & Fiser, J. Minimizing binding errors using learned conjunctive features. Neural Comput. 12, 247–278 (2000).
Grunewald, A. & Skoumbourdis, E.K. The integration of multiple stimulus features by V1 neurons. J. Neurosci. 24, 9185–9194 (2004).
Young, A.W., Hellawell, D. & Hay, D.C. Configural information in face perception. Perception 16, 747–759 (1987).
Tanaka, J.W. & Farah, M.J. Parts and wholes in face recognition. Q. J. Exp. Psychol. A. 46, 225–245 (1993).
Thompson, P. Margaret Thatcher: a new illusion. Perception 9, 483–484 (1980).
Yin, R.K. Looking at upside-down faces. J. Exp. Psychol. 81, 141–145 (1969).
Bartlett, J.C. & Searcy, J. Inversion and configuration of faces. Cogn. Psychol. 25, 281–316 (1993).
Brincat, S.L. & Connor, C.E. Underlying principles of visual shape selectivity in posterior inferior temporal cortex. Nat. Neurosci. 7, 880–886 (2004).
Valentine, T. A unified account of the effects of distinctiveness, inversion and race in face recognition. Q. J. Exp. Psychol. A. 43, 161–204 (1991).
McKone, E., Aitkin, A. & Edwards, M. Categorical and coordinate relations in faces, or Fechner's law and face space instead? J. Exp. Psychol. Hum. Percept. Perform. 31, 1181–1198 (2005).
Fraser, I.H., Craig, G.L. & Parker, D.M. Reaction time measures of feature saliency in schematic faces. Perception 19, 661–673 (1990).
Guigon, E. Computing with populations of monotonically tuned neurons. Neural Comput. 15, 2115–2127 (2003).
Kayaert, G., Biederman, I., Op de Beeck, H. & Vogels, R. Tuning for shape dimensions in macaque inferior temporal cortex. Eur. J. Neurosci. 22, 212–224 (2005).
De Baene, W., Premereur, E. & Vogels, R. Properties of shape tuning of macaque inferior temporal neurons examined using rapid serial visual presentation. J. Neurophysiol. 97, 2900–2916 (2007).
Rhodes, G. Superportraits: Caricatures and Recognition (Psychology Press Publishers, East Sussex, UK, 1996).
Webster, M.A. & MacLin, O. Figural aftereffects in the perception of faces. Psychon. Bull. Rev. 6, 647–653 (1999).
Leopold, D.A., O'Toole, A.J.O., Vetter, T. & Blanz, V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat. Neurosci. 4, 89–94 (2001).
Quiroga, R.Q., Reddy, L., Kreiman, G., Koch, C. & Fried, I. Invariant visual representation by single neurons in the human brain. Nature 435, 1102–1107 (2005).
Zhang, K. & Sejnowski, T.J. Neuronal tuning: to sharpen or broaden? Neural Comput. 11, 75–84 (1999).
Eurich, C.W. & Wilke, S.D. Multidimensional encoding strategy of spiking neurons. Neural Comput. 12, 1519–1529 (2000).
Galton, F. Composite portraits, made by combining those of many different persons into a single, resultant figure. J. Anthropol. Inst. 8, 132–144 (1879).
Salinas, E. & Thier, P. Gain modulation: a major computational principle of the central nervous system. Neuron 27, 15–21 (2000).
Liu, Y. & Jagadeesh, B. Neural selectivity in anterior inferotemporal cortex for morphed photographic images during behavioral classification or fixation. J. Neurophysiol. 100, 966–982 (2008).
Andersen, R.A., Bracewell, R.M., Barash, S., Gnadt, J.W. & Fogassi, L. Eye position effects on visual, memory, and saccade-related activity in areas LIP and 7a of macaque. J. Neurosci. 10, 1176–1196 (1990).
Zipser, D. & Andersen, R.A. A back-propagation programmed network that simulates response properties of a subset of posterior parietal neurons. Nature 331, 679–684 (1988).
Treue, S. & Martínez Trujillo, J.C. Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399, 575–579 (1999).
Koida, K. & Komatsu, H. Effects of task demands on the responses of color-selective neurons in the inferior temporal cortex. Nat. Neurosci. 10, 108–116 (2007).
Thorpe, S., Fize, D. & Marlot, C. Speed of processing in the human visual system. Nature 381, 520–522 (1996).
Keysers, C., Xiao, D.-K., Földiák, P. & Perrett, D.I. The speed of sight. J. Cogn. Neurosci. 13, 90–101 (2001).
Fabre-Thorpe, M., Richard, G. & Thorpe, S.J. Rapid categorization of natural images by rhesus monkeys. Neuroreport 9, 303–308 (1998).
Cox, D., Meyers, E. & Sinha, P. Contextually evoked object-specific responses in human visual cortex. Science 304, 115–117 (2004).
McAdams, C.J. & Maunsell, J.H.R. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J. Neurosci. 19, 431–441 (1999).
Tanaka, K., Saito, H.-A., Fukada, Y. & Moriya, M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 66, 170–189 (1991).
Fujita, I., Tanaka, K., Ito, M. & Cheng, K. Columns for visual features of objects in monkey inferotemporal cortex. Nature 360, 343–346 (1992).
Wang, G., Tanaka, K. & Tanifuji, M. Optical imaging of functional organization in the monkey inferotemporal cortex. Science 272, 1665–1668 (1996).
Bruce, C., Desimone, R. & Gross, C.G. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J. Neurophysiol. 46, 369–384 (1981).
Baylis, G.C., Rolls, E.T. & Leonard, C.M. Selectivity between faces in the responses of a population of neurons in the cortex in the superior temporal sulcus of the monkey. Brain Res. 342, 91–102 (1985).
Perrett, D.I. et al. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc. R. Soc. Lond. B 223, 293–317 (1985).
Zar, J.H. Biostatistical Analysis (Prentice Hall, Upper Saddle River, New Jersey, 1998).
Efron, B. Bootstrap methods: another look at the jackknife. Ann. Stat. 7, 1–26 (1979).
Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology (CRC Press, Boca Raton, Florida, 2007).
Acknowledgements
We are grateful to the late D. Freeman and N. Nallasamy for stimulus programming; R. Tootell, W. Vanduffel and members of the Massachusetts General Hospital monkey fMRI group for assistance with scanning; A. Dale and A. van der Kouwe for allowing us to use their multi-echo sequence and undistortion algorithm; T. Chuprina and N. Schweers for animal care and Guerbet for providing Sinerem contrast agent. This work was sponsored by US National Institutes of Health grant EY16187 and German Science Foundation grant FR 1437/3-1. D.Y.T. was supported by a Sofja Kovalevskaja Award from the Alexander von Humboldt Foundation.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Texts 1–5 (PDF 12640 kb)
Rights and permissions
About this article
Cite this article
Freiwald, W., Tsao, D. & Livingstone, M. A face feature space in the macaque temporal lobe. Nat Neurosci 12, 1187–1196 (2009). https://doi.org/10.1038/nn.2363
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2363
This article is cited by
-
Face masks are less effective than sunglasses in masking face identity
Scientific Reports (2023)
-
Local features drive identity responses in macaque anterior face patches
Nature Communications (2022)
-
Ramp-shaped neural tuning supports graded population-level representation of the object-to-scene continuum
Scientific Reports (2022)
-
A neuronal social trait space for first impressions in the human amygdala and hippocampus
Molecular Psychiatry (2022)
-
Face identity coding in the deep neural network and primate brain
Communications Biology (2022)



