Ethylenetetracarboxylic dianhydride

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H,3H-Furo[3,4-c]furan-1,3,4,6-tetrone | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6O6 | |||
| Molar mass | 168.060 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
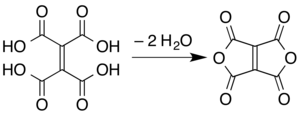
Ethylenetetracarboxylic dianhydride is a chemical compound with formula C
6O
6, that can be seen as the twofold anhydride of ethylenetetracarboxylic acid C
6H
4O
8. It has a bicyclic molecular structure consisting of two maleic anhydride rings fused by their respective alkene units. It is a pale yellow oily liquid, soluble in dichloromethane and chloroform.[1]
The compound[clarification needed] and its reactions were first reported in 1967.[2][3] More recently developed reactions for its synthesis include pyrolysis of ethylenetetracarboxylic acid[4]
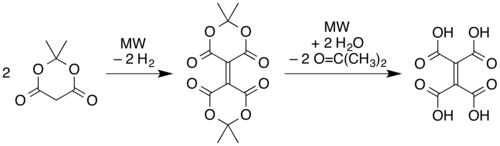
and microwave pyrolysis of solid Meldrum's acid.[1] In this latter route, two molecules of the Meldrum's acid are believed to undergo a reductive dimerization to give an alkene linkage with one "Meldrum's acid" ring on each end. The rings then open via hydrolysis of the esters to form ethylenetetracarboxylic acid, and then the carboxylic acid units recyclize with different partners (cis on each side of the alkene) as in the simple pyrolysis of the tetraacid.
An alternative dianhydride isomer, consisting of two malonic anhydride units joined by an alkene,[5] has not been reported in the literature.
See also[edit]
- Cyclohexanehexone, an elusive isomer.
References[edit]
- ^ a b Avat (Arman) Taherpour (2010), Microwave-assisted solid phase conversion study of Meldrum’s acid to ethylenetetracarboxylic dianhydride (C6O6) Spectrochimica Acta Part A, volume 75, pages 493–497 doi:10.1016/j.saa.2009.10.049
- ^ Jürgen Sauer, Barbara Schröder, Richard Wiemer (1967), Eine Studie der Diels-Alder-Reaktion, VI. Kinetischer Nachweis des Moleküls C6O6 (Dianhydrid der Äthylentetracarbonsäure). Chemische Berichte Volume 100 Issue 1, Pages 306-314 doi:10.1002/cber.19671000135
- ^ Jürgen Sauer, Barbara Schröder, Albrecht Mielert (1967), Eine Studie der Diels-Alder-Reaktion, VII. Reaktionen des Dianhydrids der Äthylentetracarbonsäure (C6O6). Chemische Berichte Volume 100 Issue 1, Pages 315-322 doi:10.1002/cber.19671000136
- ^ John M. Patterson, Nabeel F. Haidar, Loren L. Braun and Walter T. Smith, Jr. (1981), The pyrolytic behavior of ethylenetetracarboxylic acid. Journal of Analytical and Applied Pyrolysis, Volume 2, Issue 4, Pages 331-337 doi:10.1016/0165-2370(81)80005-8
- ^ CAS #160032-88-2