Abstract
Purpose
Myrrh, the oleo-gum resin of Commiphora molmol, is well known for its anti-inflammatory properties. In different animal models, it protected against DSS-, TNBS- and oxazolone-induced colitis. To date, no information concerning the effect of myrrh on barrier properties are available. Thus, this study investigates the effect of myrrh on paracellular barrier function in the absence or presence of the pro-inflammatory cytokine TNF
Methods
Monolayers of human colon cell lines HT-29/B6 and Caco-2 were incubated with myrrh under control conditions or after challenge with the pro-inflammatory cytokine TNF
Results
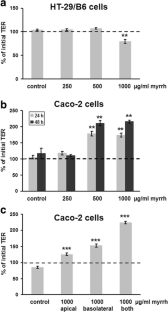
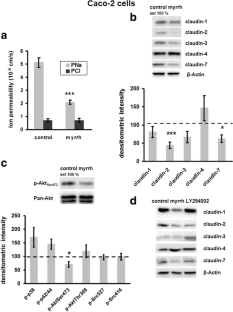
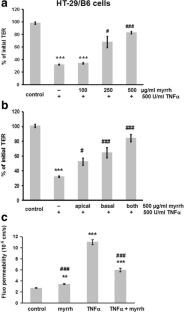
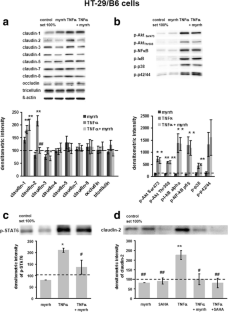
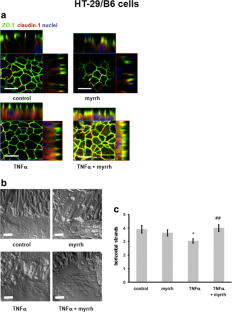
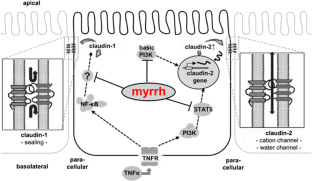
In Caco-2 cells, myrrh induced an increase in transepithelial resistance (TER) which was associated with downregulation of the channel-forming tight junction (TJ) protein claudin-2 via inhibition of the PI3 kinase signalling pathway. In HT-29/B6 cells, myrrh had no effect on barrier properties under basic conditions, but protected against barrier damage induced by TNF
Conclusions
This study shows for the first time that myrrh exerts barrier-stabilising and TNF
Similar content being viewed by others
References
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9(11):799–809
Furuse M et al (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Ikenouchi J et al (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171(6):939–945
Günzel D, Fromm M (2012) Claudins and other tight junction proteins. Compr Physiol 2(3):1819–1852
Martin-Padura I et al (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142(1):117–127
Zeissig S et al (2007) Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56(1):61–72
Heller F et al (2005) Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129(2):550–564
Oshima T, Miwa H, Joh T (2008) Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol 23(Suppl 2):S146–S150
Luettig J et al (2015) Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 3(1–2):e977176
Amasheh M et al (2008) Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr 138(6):1067–1073
Suzuki T, Hara H (2009) Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr 139(5):965–974
Amasheh M et al (2010) TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci 123:4145–4155
Luettig J, et al. (2016) The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol Nutr Food Res
Shen T et al (2012) The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 142(2):319–330
Ford RA, Api AM, Letizia CS (1992) Monographs on fragrance raw materials. Food Chem Toxicol 30 Suppl:1S–138S
Cheon JH et al (2006) Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis 12(12):1152–1161
Mencarelli A et al (2009) The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem Pharmacol 78(9):1214–1223
Manjula N et al (2006) Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-alpha, IL-1beta and IL-2. Int Immunopharmacol 6(2):122–132
Langhorst J et al (2013) Randomised clinical trial: a herbal preparation of myrrh, chamomile and coffee charcoal compared with mesalazine in maintaining remission in ulcerative colitis—a double-blind, double-dummy study. Aliment Pharmacol Ther 38(5):490–500
Langhorst J et al (2014) Distinct kinetics in the frequency of peripheral CD4+ T cells in patients with ulcerative colitis experiencing a flare during treatment with mesalazine or with a herbal preparation of myrrh, chamomile, and coffee charcoal. PLoS One 9(8):e104257
Schmitz H et al (1999) Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci 112:137–146
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96(3):736–749
Günzel D et al (2009) Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122(Pt 10):1507–1517
Rosenthal R et al (2010) Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123(11):1913–1921
Rosen MJ et al (2011) STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis 17(11):2224–2234
Amasheh S et al (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976
Furuse M et al (2001) Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153(2):263–272
Thongon N, Krishnamra N (2012) Apical acidity decreases inhibitory effect of omeprazole on Mg(2+) absorption and claudin-7 and -12 expression in Caco-2 monolayers. Exp Mol Med 44(11):684–693
Kreusel KM et al (1991) Cl− secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Phys 261(4):574–582
Cui W et al (2010) Tumor necrosis factor alpha increases epithelial barrier permeability by disrupting tight junctions in Caco-2 cells. Braz J Med Biol Res 43(4):330–337
Gitter AH et al (2000) Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-alpha. Ann N Y Acad Sci 915:193–203
Mankertz J et al (2009) TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 336(1):67–77
Epple HJ et al (2009) Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 58(2):220–227
Schumann M, et al. (2011) Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut
Prasad S et al (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Investig 85(9):1139–1162
Suzuki T, Yoshinaga N, Tanabe S (2011) Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 286(36):31263–31271
Rosen MJ et al (2013) STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. J Immunol 190(4):1849–1858
Dhawan P et al (2011) Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene 30(29):3234–3247
Escaffit F, Boudreau F, Beaulieu JF (2005) Differential expression of claudin-2 along the human intestine: implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol 203(1):15–26
Holmes JL et al (2006) Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 6(6):581–588
Mankertz J et al (2004) Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun 314(4):1001–1007
Milatz S et al (2010) Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta 1798(11):2048–2057
Piontek J et al (2011) Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci 68(23):3903–3918
McCarthy KM et al (2000) Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci 113 Pt 19:3387–3398
de Oliveira SS et al (2005) Claudins upregulation in human colorectal cancer. FEBS Lett 579(27):6179–6185
Schmitz H et al (2000) Epithelial barrier and transport function of the colon in ulcerative colitis. Ann N Y Acad Sci 915:312–326
Das P et al (2012) Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch 460(3):261–270
Albrecht U et al (2014) Efficacy and safety of a herbal medicinal product containing myrrh, chamomile and coffee charcoal for the treatment of gastrointestinal disorders: a non-interventional study. BMJ Open Gastroenterol 1(1):e000015
Abdul-Ghani RA, Loutfy N, Hassan A (2009) Myrrh and trematodoses in Egypt: an overview of safety, efficacy and effectiveness profiles. Parasitol Int 58(3):210–214
Deng R (2007) Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovasc Drug Rev 25(4):375–390
Acknowledgements
The superb technical assistance of Britta Jebautzke and In-Fah Lee is gratefully acknowledged. The authors thank Dr. Domenica Hamisch for carefully proofreading our manuscript and valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by grants from Deutsche Forschungsgemeinschaft (FOR 721), Sonnenfeld-Stiftung Berlin and Repha GmbH (Hannover, Germany). Myrrh was provided by Repha GmbH.
Conflict of interest
R.R., J.L., N.A.H., S.M.K., U.A., M.F. and J.D.S. declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Rosenthal, R., Luettig, J., Hering, N.A. et al. Myrrh exerts barrier-stabilising and -protective effects in HT-29/B6 and Caco-2 intestinal epithelial cells. Int J Colorectal Dis 32, 623–634 (2017). https://doi.org/10.1007/s00384-016-2736-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2736-x