Biomarkers in a socially exchanged fluid reflect colony maturity, behavior, and distributed metabolism
Abstract
In cooperative systems exhibiting division of labor, such as microbial communities, multicellular organisms, and social insect colonies, individual units share costs and benefits through both task specialization and exchanged materials. Socially exchanged fluids, like seminal fluid and milk, allow individuals to molecularly influence conspecifics. Many social insects have a social circulatory system, where food and endogenously produced molecules are transferred mouth-to-mouth (stomodeal trophallaxis), connecting all the individuals in the society. To understand how these endogenous molecules relate to colony life, we used quantitative proteomics to investigate the trophallactic fluid within colonies of the carpenter ant Camponotus floridanus. We show that different stages of the colony life cycle circulate different types of proteins: young colonies prioritize direct carbohydrate processing; mature colonies prioritize accumulation and transmission of stored resources. Further, colonies circulate proteins implicated in oxidative stress, ageing, and social insect caste determination, potentially acting as superorganismal hormones. Brood-caring individuals that are also closer to the queen in the social network (nurses) showed higher abundance of oxidative stress-related proteins. Thus, trophallaxis behavior could provide a mechanism for distributed metabolism in social insect societies. The ability to thoroughly analyze the materials exchanged between cooperative units makes social insect colonies useful models to understand the evolution and consequences of metabolic division of labor at other scales.
eLife digest
Division of labor is essential for cooperation, because groups can achieve more when individuals specialize in different tasks. This happens across the natural world, from different cells in organisms performing specific roles, to the individuals in an ant colony carrying out diverse duties. In both of these systems, individuals work together to ensure the survival of the collective unit – the body or the colony – instead of competing against each other. One of the main ways division of labor is evident within these two systems is regarding reproduction. Both in the body and in an ant colony, only one or a few individual units can reproduce, while the rest provide support. In the case of ant colonies, only queens and males reproduce, while the young workers nurse the brood and older workers forage for food.
This intense cooperation requires close communication between individual units – in the case of some species of ants, by sharing fluids mouth-to-mouth. These fluids contain food but also many molecules produced by the ants themselves, including proteins. Given that both individuals and the colony as a whole change as they age – with workers acquiring new roles, and new queens and males only reared once the colony is mature – it is likely that the proteins transmitted in the fluid also change.
To better understand whether the lifecycles of individuals and the age of the colony affect the fluids shared by carpenter ants Camponotus floridanus, Hakala et al. examined the ant-produced proteins in these fluids. This revealed differences in the proteins shared by young and mature colonies, and young nurse ants and older forager ants. In young colonies, the fluids contained proteins involved in fast sugar processing; while in mature colonies, the fluids contained more proteins to store nutrients, which help insect larvae grow into larger individuals, like queens. Young worker ants, who spend their time nursing the brood, produced more anti-aging proteins. This may be because these ants are in close contact with the queen, who lives much longer than the rest of the ants in the colony. Taken together, these observations suggest that ants divide the labor of metabolism, as well as work and reproduction.
Dividing the labor of metabolism among individuals is one more similarity between ants and the cells of a multicellular organism, like a fly or a human. Division of labor allows the sharing of burden, with some individuals lightening the load of others. Understanding how ants achieve this by sharing fluids could shed new light on this complex exchange at other scales or in other organisms. By matching proteins to life stages, researchers have a starting point to examine individual molecules in more detail.
Introduction
In the course of social evolution, related organisms have formed cooperative entities such as multicellular organisms or groups of social animals (Szathmáry, 2015; Queller, 1992; Hamilton, 1963). In social animal groups, collective decisions on movement, reproduction and even development are needed for survival (Miller et al., 2013; Couzin, 2009). Some social groups have taken this coordination to a very high level: social insect societies develop and function as a single unit instead of as competing individuals, as ‘superorganisms’ paralleling the development of multicellular organisms as a single unit rather than as a set of competing cells (Johnson and Linksvayer, 2010; Boomsma and Gawne, 2018).
In these superorganismal societies, reproductive queens and males function as the germline, and workers as the soma. Similarly to different tissues in multicellular organisms, workers can be further specialized and exhibit division of labor across different behavioral and morphological castes (Bourke, 2011). While morphological castes are determined during development, the behavioral caste of an individual worker typically changes during its lifetime. At the beginning of their adult life, workers specialize inside the nest as nurses focusing on brood care, and as they age, they switch to foraging outside of the nest (Huang and Robinson, 1996). Social insect colonies also go through life stages. Young colonies have an initial growth phase where they solely produce one type of worker, and only later in their life cycle they may produce more specialized worker castes and finally, males and queens (Wilson, 1971). The switch to reproductive phase is a major life-history transition at the colony level, and connected to female caste determination. In social Hymenoptera, determination of whether a female larva develops into a queen or a worker, and what kind of worker exactly, is controlled by intricate differences of gene expression of the same female genome, guided primarily by environmental factors, in particular nutrition and social cues, sometimes partially influenced by genetics (Wheeler, 1986; Anderson et al., 2008; Rajakumar et al., 2018; Schwander et al., 2010).
Coordinated function of tightly integrated groups such as social insect colonies, and subgroups such as their different castes, has been described as social physiology (Friedman et al., 2020), consisting of various behavioral, morphological, and molecular mechanisms that ensure cooperation and inclusive fitness benefits for all group members. As a part of their social physiology, some social insect societies have developed a form of social circulatory system (Wheeler, 1928), where nutrition and endogenously produced functional molecules, such as hormones, are transferred mouth-to-mouth from the foregut of one individual to another (LeBoeuf et al., 2016; LeBoeuf et al., 2018). This social fluid transfer is called stomodeal trophallaxis (Meurville and LeBoeuf, 2021). It ensures not only that food is distributed to all adults and larvae within the colony, but also that all individuals of the colony are interconnected through shared bodily fluids. Trophallactic fluid of ants and bees typically contains endogenous proteins involved in digestion, immune defense and developmental regulation (LeBoeuf et al., 2016), indicating that this fluid transmits more than food.
Molecular signals are important in controlling the colony life histories and guiding caste determination both at the colony level and at the individual level. Queen pheromones are central signaling molecules acting across individuals (Kocher and Grozinger, 2011; Nijhout and Wheeler, 1982; Pamminger et al., 2016). Juvenile hormone and vitellogenin are central signaling molecules in classical insect development that may also play across-individual roles in some social insects (LeBoeuf et al., 2016; LeBoeuf et al., 2018; Scharf et al., 2007; Harwood et al., 2019). Together with fundamental nutrient-response signaling pathways (insulin, TOR), these molecules establish the developmental trajectories of individuals (Chandra et al., 2018; Libbrecht et al., 2013). In solitary organisms, such molecules are produced and function solely within the organism’s own body. In contrast, in social Hymenoptera even the molecules traditionally functioning within-individuals can be secreted to the crop and distributed among the society members through trophallaxis and the social circulatory system (LeBoeuf et al., 2016).
Molecular components transmitted through trophallaxis, namely juvenile hormone and juvenile hormone esterase-like proteins, have been shown to influence the development of ant larvae (LeBoeuf et al., 2016; LeBoeuf et al., 2018). Thus, it is possible that molecules in trophallactic fluid may influence caste determination, similarly to honeybee workers feeding larvae with royal jelly to direct their development toward a queen fate (LeBoeuf et al., 2016; Buttstedt et al., 2014; Buttstedt et al., 2016; Kamakura, 2011; Kucharski et al., 2015). The molecular functions of trophallactic fluid are still largely unstudied, but it is known, for example, that social isolation changes its composition (LeBoeuf et al., 2016), with some protein components of this fluid shifting with social environment. In medicine, such correlations are typically used to define biomarkers for specific conditions and treatments, and often both accurately predict function and provide mechanistic insights (Strimbu and Tavel, 2010). We propose that trophallactic fluid could both reflect and affect the social environments of the colony, thus providing important cues for collective decision making. However, it is not yet feasible to study the causes and consequences of the molecular composition of trophallactic fluid, as it is still largely unknown how much and what kind of qualitative and quantitative variation is present.
If indeed trophallactic fluid acts as a form of social circulatory system, managing distributed metabolic processes related to colony maturation, endogenously produced factors should correlate with colony life stages. To test this, we analyzed the trophallactic fluid proteome of the carpenter ant Camponotus floridanus at different scales. Our aim is to demonstrate that trophallactic fluid proteomes are filled with biomarkers reflecting biotic and abiotic conditions at both the colony and individual scale.
Results
We sought to determine whether the endogenously produced proteins present in trophallactic fluid create a robust biomarker-like signature of colony status. To assess this, we analyzed the trophallactic fluid proteomes of colonies at different stages in the colony life-cycle (Young vs. Mature), of colonies in natural conditions or kept in the lab (Field vs. Lab), and between colonies found on different nearby islands (East vs. West) (Figure 1; Supplementary file 1). Because trophallactic fluid proteins may be differentially expressed, transmitted, and/or sequestered across the social network of a colony, we also analyzed trophallactic fluid proteomes of single individuals in different colony ‘tissues’ – in-nest workers taking care of brood and out-of-nest workers (Nurse vs. Forager).
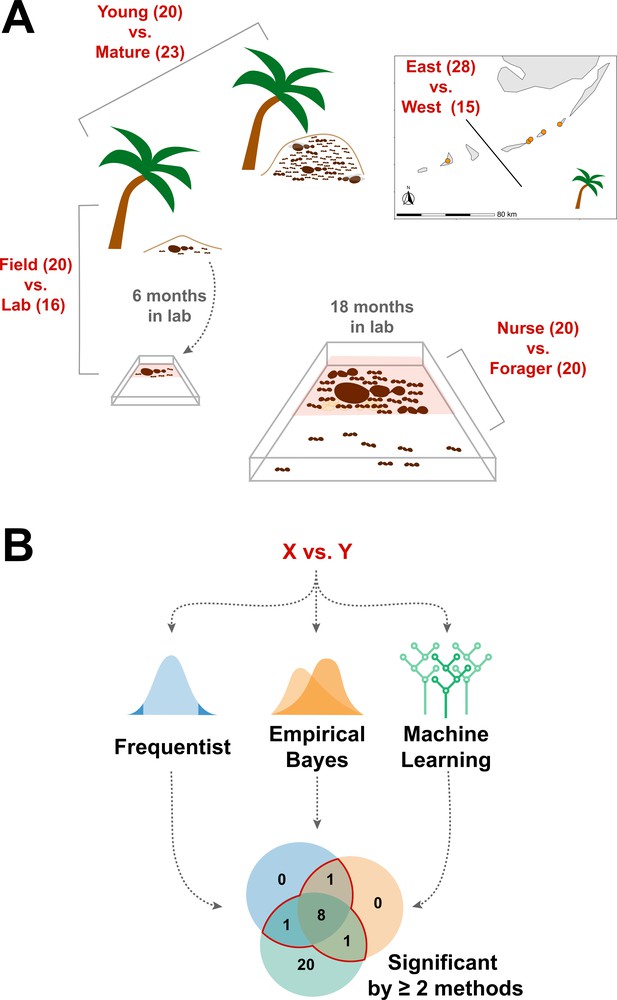
Schematic of study design.
(A) Four comparisons, Young vs. Mature, Nurse vs. Forager, Field vs. Lab, and East vs. West, analyzed in this study with sample numbers indicated in parentheses. In all comparisons sample numbers indicate colonies with the exception of Nurse vs. Forager, where samples are from single individuals, ten each from four colonies. Palm trees indicate field samples and boxes indicate laboratory samples. (B) Schematic of analysis approach to find robustly differing proteins in each comparison. Sample information can be found in Supplementary file 1.
Overall proteome variation
Over the 73 colony and 40 single-individual trophallactic fluid samples analyzed, a total of 519 proteins were identified (Figure 2). Trophallactic fluid samples contained a set of 27 ‘core’ trophallactic fluid proteins that were present in all samples regardless of life-cycle, life-stage or environmental conditions. Fifty-seven percent of the 519 proteins, we observed were present in less than half of the samples. Even though the most common proteins displayed higher average abundance, across the entire dataset, protein abundance did not correlate with the proportion of samples containing the protein – even proteins present in only a small proportion of the samples in some cases exhibited high abundance (Figure 2—figure supplement 1). The overall protein abundance was higher in colony samples relative to single individual samples, reflective of the larger trophallactic fluid volume collected. The number of proteins identified for a given sample correlated with trophallactic fluid sample volume (Pearson correlation test p < 0.03, r = 0.24 for colony samples and p < 0.01, r = –0.40 for single-individual samples).
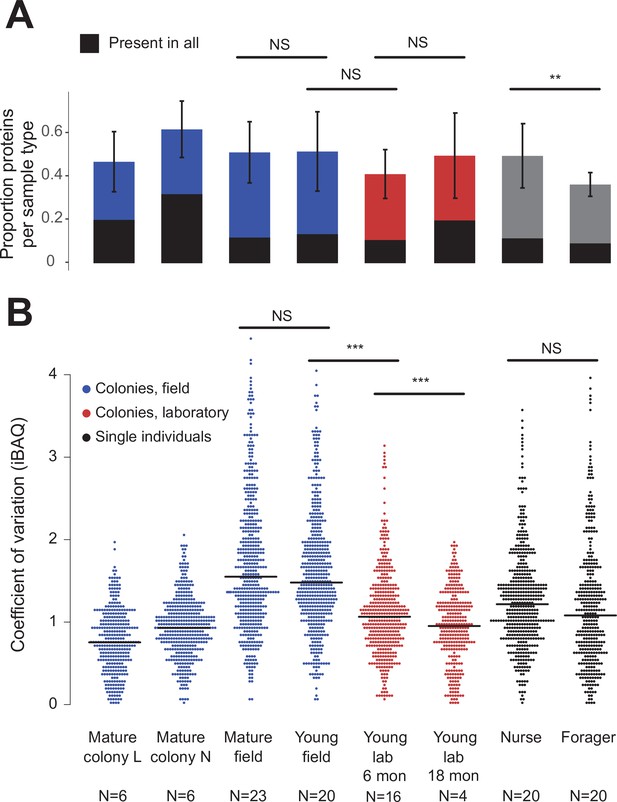
Protein presence in trophallactic fluid varies with biotic and abiotic factors.
(A) Mean ± SD of the proportion of proteins present in samples of a given type. Proportion of proteins present in all samples of a given type are highlighted in black. (B) Coefficient of variation (standard deviation/mean), calculated for the iBAQ values greater than zero of all the proteins identified by sample type. Sample sizes per type are given under their names. Mature L and Mature N are mature colonies that were sampled six times to assess within-colony variation in colony samples. Significance of comparisons based on gamma GLM (A) or negative binomial GLM (B): NS indicated when p > 0.05 significant, ** p < 0.01, *** p < 0.001 (full results in Figure 2—source data 1; Figure 2—source data 2).
-
Figure 2—source data 1
Coefficient of variation by sample type Post-hoc comparisons of gamma GLM on coefficient of variation by sample type.
- https://cdn.elifesciences.org/articles/74005/elife-74005-fig2-data1-v3.pdf
-
Figure 2—source data 2
Protein number by sample type Post-hoc comparisons of negative binomial GLM on protein number explained by sample type.
- https://cdn.elifesciences.org/articles/74005/elife-74005-fig2-data2-v3.pdf
Field-collected samples exhibited more variable proteomes than did lab-collected samples (Figure 2b, gamma GLM posthoc p-values < 0.001, Figure 2—source data 1). Further, colonies that had been in the lab for more than one year showed less variable proteomes than did colonies that had been in the lab for only six months (Figure 2b, gamma GLM z = −4.46, SE = 0.04, p < 0.001). The trophallactic proteome variability of young and mature colonies did not differ significantly, nor did nurses’ and foragers’ (Figure 2b). Foragers had fewer identified proteins in their trophallactic fluid than did nurses (Figure 2a, negative binomial z = 3.72, SE = 0.08, p = 0.005), and there were no significant differences among the main full colony samples (Figure 2—source data 2).
When the principal components of the trophallactic fluid proteomes were analyzed, the samples tended broadly to align with others of the same type, although clusters were not fully distinct (Figure 3A and B). We developed a metric, self-similarity (S), to assess the depth of difference within and across sample types (Figure 3C). Because field-collected samples had more diverse protein content even within sample types (Figures 2 and 3A), the self-similarity in the Young vs. Mature comparison is low (Figure 3C). Single individual samples, and especially forager samples were less complex, allowing a larger proportion of their dissimilarity to be explained by sample type. Further, because our classification of nurse and forager is based on the individuals’ location on brood or out-of-nest, it is possible that some nurse-classified individuals were either misclassified, transitioning from nurse to forager, or had trophallactic fluid in their crop uncharacteristic of their behavioral caste.
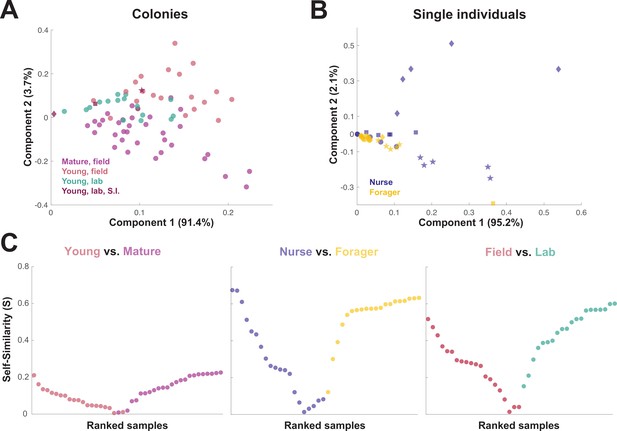
Similarity across trophallactic fluid proteome samples of colonies and single individuals.
Principal component analysis for all proteins for (A) colony samples and (B) single individual samples from the four colonies. Symbols representing the four colonies represented in (B) can be found in maroon in (A). (C) Ranked Self-similarity S for each sample type comparison. Self-similarity is the absolute value of the difference between dissimilarity within and across samples divided by the average dissimilarity of all samples (by standardized Euclidean distance of protein abundance). Samples with higher S are more similar to samples of the same type, while samples with an S of zero are equidistant to the centroids of the two sample groups.
-
Figure 3—source code 1
Matlab source code to produce self-similarity scores, plots and PCA plots that make up Figure 3, https://github.com/dradri/variation2021.
- https://cdn.elifesciences.org/articles/74005/elife-74005-fig3-code1-v3.zip
-
Figure 3—source data 1
Matlab MAT data file based on iBAQ values, gene names, and sample classes to produce self-similarity scores, plots and PCA plots that make up Figure 3.
- https://cdn.elifesciences.org/articles/74005/elife-74005-fig3-data1-v3.mat
Comparisons of trophallactic fluid across conditions
In addition to characterizing the most abundant and core proteins of the trophallactic fluid (Figure 4, Figure 4—figure supplement 1), we wanted to robustly identify proteins that differ significantly in our comparisons despite the noise inherently present in this social fluid. To accomplish this, we chose to overlay three distinct statistical approaches (Figure 1B): classical frequentist, empirical Bayes and machine-learning in the form of random forest classification. In our main comparisons, Young vs. Mature colonies from the field, young colonies in the Field vs. Lab, and individual Nurses vs. Foragers in the lab, we found significant differences between groups with all three analysis methods (Figure 5, Figure 5—figure supplement 1, Figure 5—figure supplement 2, full results for the significantly differing proteins in Supplementary file 2 and for all proteins in Supplementary files 3-5).
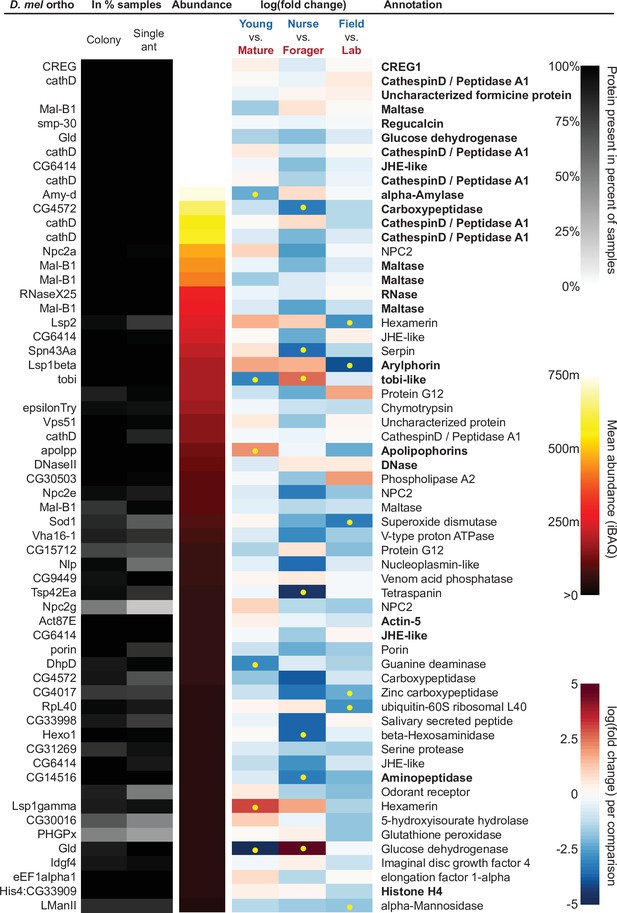
The sixty most abundant proteins in trophallactic fluid over 73 colony and 40 single individual samples.
Ranking of abundance (including missing values). From left to right, Drosophila melanogaster orthologs, proportion of samples in which the protein was identified in colony samples and single individual samples, average iBAQ abundance across all samples, log2 of the fold change in abundance between types for a given comparison, the comparisons for which the protein was significant in two out of three methods are marked with yellow dots, annotation terms. Annotation terms are bolded for the 25 out of 27 core trophallactic fluid proteins that are amongst the 60 most abundant proteins. The additional but less abundant core proteins are a cathepsin (26–29 p) and a myosin heavy chain (Mhc). For protein accession numbers, see Figure 4—figure supplement 1.
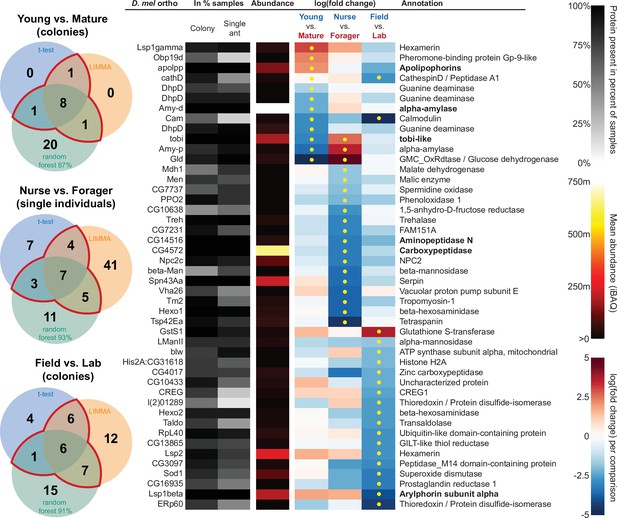
All proteins that significantly differ in two out of three of the analysis methods (frequentist, empirical Bayes, and random forest classification with SHAP values).
From left to right, Venn diagrams of significance overlap between methods, Drosophila melanogaster orthologs, proportion of samples in which the protein was identified in colony samples and single individual samples, average iBAQ abundance across all samples calculated without missing values, log2 of the fold change in abundance between types for a given comparison, the comparisons for which the protein was significant in two out of three methods are marked with yellow dots, annotation terms. Annotation terms are in bold for the core trophallactic fluid proteins present in all samples. For visualization of each analysis method, see Figure 5—figure supplement 1. For protein accession numbers, see Figure 5—figure supplement 2. For all the 135 proteins significantly differing in any analysis, see Supplementary file 2. For full model results, see Supplementary files 3-5.
-
Figure 5—source code 1
Jupyter notebook to run random forest analyses, https://github.com/dradri/variation2021.
- https://cdn.elifesciences.org/articles/74005/elife-74005-fig5-code1-v3.zip
For the Young vs. Mature comparisons, there were 10, 10, and 30 differentially abundant proteins according to frequentist t-test, empirical Bayesian LIMMA and the random forest approach, respectively. Similarly, for the Nurse vs. Forager comparison there were 21, 57, and 26 differentially abundant proteins, and when young colonies were brought to the laboratory and resampled after six months, 17, 31, or 29 proteins had significantly different abundance. The average accuracies of classification for comparisons with the random forest approach were: Young vs. Mature, 87 %; Nurse vs. Forager, 93 %; and Field vs. Lab, 91 %. This indicates that our trained classifier can predict whether a trophallactic fluid sample originates from a nurse or a forager with 93 % accuracy. We found no clear signature of spatial structure (East vs. West) in the trophallactic fluid proteomes. The frequentist analysis between different sampling areas found no significantly different proteins, and the random forest model did not reach high enough accuracy for this dataset to be informative (58 % classification accuracy). Only the empirical Bayes approach found eight proteins that significantly differed between the sampling areas (Figure 5—figure supplement 1, Supplementary files 2 and 4).
To leverage the unique benefits of the different forms of analysis, we focused our further analyses on proteins significantly different in two out of the three forms of analysis. Here, young and mature colonies differed by 12 proteins, and nurses and foragers differed by 19 proteins (Figure 5). When young colonies were brought to the laboratory and resampled six months later, the trophallactic fluid proteomes differed significantly by 20 proteins. Additionally, the single individual dataset showed that proteomes are affected both by colony of origin and by behavioral role of the individual, with 60 proteins showing significant interaction between the two factors (Supplementary file 3).
Functions of the proteins in trophallactic fluid
To investigate the functions of the proteins found in trophallactic fluid, we performed functional enrichment analysis of gene ontology terms, pathways and protein-protein interaction (PPI) networks of the trophallactic fluid proteins’ Drosophila melanogaster orthologs. The 60 most abundant proteins in trophallactic fluid (Figure 4, Figure 4—figure supplement 1) are predominantly involved in the biological processes of carbohydrate metabolism, lipid and sterol transport (Figure 6, Figure 6—source data 1, FDR < 0.00038, FDR < 0.0013 and FDR < 0.0087 respectively). The larval serum protein complex was represented by three out of four members in both the most abundant proteins and in the significantly differing proteins (hexamerins/arylphorins: Lsp1beta, Lsp1gamma, and Lsp2). A strong representation of the innate immune system (Reactome pathway FDR < 6.57e-5) was evident as were lysosomal processes (KEGG pathway, FDR < 3.21e-9).
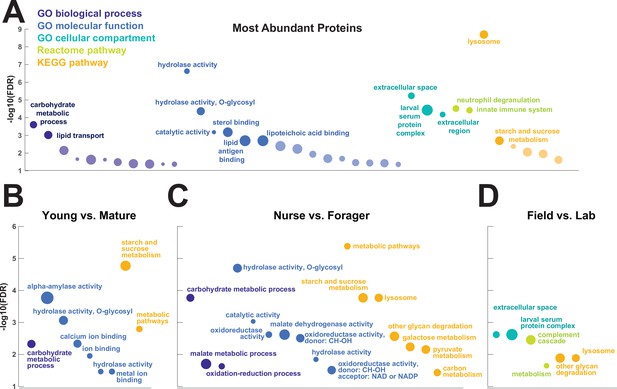
Gene set enrichment analysis of trophallactic fluid.
Significant terms for Drosophila melanogaster orthologs of (A) the 60 most abundant trophallactic fluid proteins, trophallactic fluid proteins significantly differing between (B) Young vs. Mature, (C) Nurse vs. Forager, and (D) Field vs. Lab, with -log10(FDR) indicated on y-axes. Deep purple indicates GO biological process; blue, GO molecular function; turquoise, GO cellular compartment; lime green, Reactome pathway; orange, KEGG pathway. Circle size indicates strength, log10(observed proteins / expected proteins in a random network of this size). Full results can be found in Figure 6—source data 1.
-
Figure 6—source data 1
Gene set enrichment analyses of trophallactic fluid proteins.
Sheet 1 provides the network characteristics for all gene set enrichment analyses. The detailed results for each network are presented in sheet 2 for the 60 most abundant proteins, and in sheet 3 for the trophallactic fluid proteins significantly differing in two out of three of our statistical methods, first combined and then separately for the three main comparisons. In each case, analysis was performed on D. melanogaster orthologs of C. floridanus proteins. Observed gene count indicates how many proteins in the network are annotated with the term. Background gene count indicates how many proteins in total have this term, in this network and in the background. Strength describes how large the enrichment effect is: log10(observed proteins / expected proteins in a random network of this size). False Discovery Rate describes how significant the enrichment is. p-Values are corrected for multiple testing within each category using the Benjamini– Hochberg procedure. The significant annotations are indicated for GO: Biological process (GO:BP), GO: Molecular function (GO:MF), GO: Cellular component (GO:CC), Reactome pathways and KEGG pathways.
- https://cdn.elifesciences.org/articles/74005/elife-74005-fig6-data1-v3.xlsx
Beyond the 60 most abundant proteins in trophallactic fluid, many others are of interest as well. A critical protein in insect physiology, vitellogenin is the 93rd most abundant protein in trophallactic fluid, present in 77% and 88% of colony and single individual samples, respectively. Three of the 60 most abundant proteins had no similarity to Drosophila genes, and thus could not be included in the functional enrichment analysis. One of them is a putative odorant receptor, another a G-protein alpha subunit, and the third showed no orthology to characterized proteins. None of these proteins significantly differed in more than one analysis for a given comparison.
Many of the trophallactic fluid proteins, abundant or significantly differing, were represented in trophallactic fluid by multiple genes from the same protein family, in some cases part of tandem repeats in the genome, indicative of relatively recent evolution. Multiple proteins of the same family were found in the most abundant trophallactic fluid proteins (Figure 4, Figure 4—figure supplement 1): a family of cathepsinD-like proteins (six in the top 60; LeBoeuf et al., 2016; Hamilton et al., 2011) and a family of Maltase-B1-like proteins (five in the top 60). In the list of significantly differing proteins (Figure 5, Figure 5—figure supplement 2), we observed fewer members of these families and instead saw three guanine deaminase proteins, all of which significantly differed in the Young vs. Mature comparison. Other families that showed duplications were glucose dehydrogenases, CREG1 and tobi-like proteins (target-of-brain-insulin).
There was an overlap of 16 proteins between the most abundant proteins and the proteins significant in two out of three of our statistical methods in any of the comparisons. The PPI network for our differentially abundant protein set (46 proteins, Figure 5) was similar to that of the most abundant proteins (Figure 4) but with increased interaction in the networks of the proteins themselves beyond what would be expected by chance (PPI enrichment p-value < 2.35e-11 in differentially abundant proteins relative to p-value < 1.59e-9 in abundant proteins), with noted enrichment in oxidation-reduction processes (FDR < 0.0026) and stronger enrichment in carbohydrate metabolic processes (FDR < 2.15e-6).
To better understand the functions of the significantly differing proteins in each comparison, we analyzed the GO terms and PPI networks of proteins significant in two out of three statistical methods separately for each of our three main comparisons (Figure 6, Figure 6—source data 1). The Nurse vs. Forager comparison yielded a network of proteins with more interaction than would have been predicted by chance (PPI enrichment p-value < 2.57e-4) as well as a higher degree of PPI enrichment than the other two comparisons (Young vs. Mature p < 0.002 and Field v Lab p < 0.02). The orthologs of differentially abundant proteins found in the behavioral caste comparison involved not only carbohydrate processing (FDR < 1.7e-4), but also oxidation-reduction and malate metabolic processes (FDR < 0.023 and FDR < 0.02, respectively). These pathways have been implicated in the determination of lifespan (Wiley and Campisi, 2016). Indeed, two of the 46 differentially abundant proteins over all comparisons have D. melanogaster orthologs with the gene ontology term ‘determination of adult lifespan’ (Men, Sod1). The C. floridanus tetraspanin, significantly more abundant in nurse trophallactic fluid, is a one-to-many ortholog to the family of Tsp42E genes, one of which has also been implicated in determination of adult lifespan in D. melanogaster.
As trophallactic fluid samples of young and mature colonies were distinguishable by principal component analysis and our random forest classifier, we wanted to see if our trained classifier could assess a change in maturity of our young colonies after they had spent six months in the laboratory. Our random forest classifier assigned an average out-of-box maturity score to our 16 laboratory samples of 42 % mature, reflecting the intermediate position of the laboratory colony samples in Figure 3.
Discussion
When an ant colony matures, the protein composition of trophallactic fluid changes in biomarker-like manner, suggesting that these proteins circulating amongst individuals play a role in age-related colony metabolism and physiology. At the individual level, certain trophallactic fluid proteins correlate with behavioral caste within the colony, a trait known to encompass both individual task requirements and age (Korb et al., 2021; Mersch et al., 2013; Wild et al., 2021). Trophallactic fluid complexity declines over time when colonies are brought from the field to the laboratory. This may reflect dietary, microbiome or environmental complexity – typical of traits that have evolved to deal with environmental cues and stressors (e.g. immunity, Lazzaro and Little, 2008).
Overall, our data reveal a rich network of trophallactic fluid proteins connected to the principal metabolic functions of ant colonies and their life cycle. Pinpointing contexts that induce changes in trophallactic fluid, along with the exact targets and functions of the proteins, are important subjects for future work. Our establishment of biomarkers transmitted over the social circulatory system that correlate with social life will allow researchers to formulate and test hypotheses on these proteins’ functional roles.
Metabolism changes with maturity
We found that trophallactic fluid includes many enzymes involved in metabolism and protein products of metabolism. Many are core trophallactic fluid proteins present in all samples, but many also differ significantly among the colony and individual life stages. Some proteins abundant in mature colonies (Lsps, apolpp Burmester and Scheller, 1999; Burmester, 2002; Burmester, 1999) are major insect nutrient storage proteins (Burmester, 1999) that may be required to consolidate resources into large workers and sexuals, potentially acting as superorganismal hormones. Proteins abundant in foragers and young colonies (Gld, tobi, Amy, Mal, Buch et al., 2008; White et al., 2021) are well-conserved enzymes for fast sugar processing. This suggests a functional role of trophallactic fluid in the social physiology of ant colonies.
Similar shifts in protein composition or gene expression can be seen in different tissues of multicellular organisms as life-stage priorities change, for example in the midgut of drosophila females after mating, where changes in expression are observed in many genes orthologous to the proteins we found here (White et al., 2021). Additionally, Drosophila larval hemolymph proteome changes as development unfolds (Handke et al., 2013), and many of these same proteins also appear in our comparisons of worker trophallactic fluid. We suggest that regulation of larval development may at least in part occur over the social network of ants, in line with previous experimental results (LeBoeuf et al., 2018).
Ageing and division of metabolic labor
Viewing the colony as a superorganism, the division of reproductive labor between different types of workers (soma) and queens (germline) should result in different individuals requiring differing resources and sustaining differing metabolic costs. Our results support this hypothesis. We show that trophallactic fluid transmits numerous factors linked to ageing and coping with oxidative stress, including two of the three most well-known antioxidant enzymes: superoxide dismutase and glutathione peroxidase (Monaghan et al., 2009). These and other ageing-related proteins, such as those in redox pathways and malate metabolism (Wiley and Campisi, 2016; Koch et al., 2021), are especially elevated in nurses, the individuals that are physically the closest to the brood and queen in the trophallactic network.
These results link trophallactic fluid to one of the main topics of evolutionary ecology: the longevity-fecundity tradeoff between reproduction and coping with oxidative stress (Monaghan et al., 2009; Flatt, 2011; Edward and Chapman, 2011). Social insect individuals seemingly escape this tradeoff with long-lived and highly reproductive queens and short-lived, non-reproductive workers (Monaghan et al., 2009; Edward and Chapman, 2011; Heinze and Schrempf, 2008). We reveal a possible distributed metabolism which could explain why social insects seem to subvert this tradeoff. If molecules dealing with oxidative stress, or beneficial products of metabolism (nutrient storage proteins) can be spread over the circulatory system, as our results show, certain individuals may bear the costs that others in the network incur. This could account for some of the puzzling results on the plasticity of senescence in social insects (Kramer et al., 2021; Heinze and Giehr, 2021; Lucas et al., 2019), and provides a new perspective to analyze the regulatory changes of social insect reproductive castes with regard to ageing (Korb et al., 2021; Negroni et al., 2019; Elsner et al., 2018; Corona et al., 2005; Gstöttl et al., 2020; Corona et al., 2016; von Wyschetzki et al., 2015). While most previous work has focused almost exclusively on gene expression, we show that for species that engage in trophallaxis, expression studies are necessary but insufficient to understand where in the colony the relevant genes act.
Our gene-set enrichment analysis showed significant enrichment in immunity-related proteins characteristic of phagocytic hemocytes (Shokal and Eleftherianos, 2017) in trophallactic fluid (‘innate immune system’, ‘complement cascade’, ‘neutrophil degranulation’). These results indicate that hemocytes may themselves be transmitted mouth-to-mouth, and generally shows the involvement of the social circulatory system in colony-level immune responses with implications for social immunity. Our results do not show clear caste differentiation in the abundance of immune-related proteins, as did a study in honey bees in glands that produce trophallactic fluid proteins (Vannette et al., 2015), though we do see similar regulation of sugar processing enzymes and glutathione-S-transferases.
Evolution of trophallactic fluid
Trophallactic fluid is one of many social fluids in biology – milk and seminal fluid are similar examples of direct transfers of biological material between individuals. Such socially exchanged materials often contain molecules that target receivers’ physiology beyond the fundamental reason for the transfer (Bromfield et al., 2014; Savino et al., 2013), and allow social effects to directly influence the evolutionary process as indirect genetic effects (Linksvayer, 2015; McGlothlin et al., 2010; Wolf et al., 1998). Some of the proteins we find to be significantly differing in our comparisons have previously been implicated in these other social transfers. For example, one of our protein hits is orthologous to Drosophila’s CG10433, a seminal fluid protein (Findlay et al., 2008) that impacts juvenile-hormone-associated hatch-rate post-mating (Liu et al., 2014). In another parallel to a phylogenetically distant social fluid, trophallactic fluid’s most abundant protein is CREG1, a secreted growth-associated glycoprotein also abundant in mammalian milk (Zhang et al., 2015). Finding molecular parallels in distinct behavioral processes hints at the fundamental role of these exchanges in the evolution of social physiology, and possibly common adaptive requirements for bioactive social fluids.
Lysosomal pathways are enriched in our most abundant trophallactic fluid proteins and in our set of significantly varying trophallactic fluid proteins between nurses and foragers, according to the KEGG analysis. Lysosomes are acidic and can be major players in secretion, autophagic flux and exocytosis (Tancini et al., 2020; Csizmadia et al., 2018; Maruzs et al., 2019) – processes that may be important for nurses that feed larvae by trophallaxis. These significant lysosomal signatures we see in trophallactic fluid may indicate the mechanism of secretion (Martínez et al., 2020), or may give us cues of how this fluid has evolved. As trophallactic fluid has become acidified in formicine ants (Tragust et al., 2020), lysosomal genes could have been duplicated and neofunctionalized to a new role in this acidic fluid, similarly to juvenile-hormone-esterase-like proteins in trophallactic fluid (LeBoeuf et al., 2018). The fact that many abundant trophallactic fluid proteins represent clusters of related proteins from a few families (cathepsins, guanine deaminases, maltases) suggests there has been adaptive evolution in the proteins arriving in this fluid.
Conclusions
We show that the protein composition of ant trophallactic fluid varies across different external contexts and internal conditions both at the colony and at the individual level, suggesting that the dynamic trophallactic fluid proteome has key functions in social physiology and life cycle of colonies. By describing the natural variation of trophallactic fluid we have laid the groundwork for future studies on the possible functions of these proteins in controlling the colony life cycle, senescence, and behavior.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Other | UniProt Reference proteome (Camponotus floridanus); accessed February 2020 | UniProt | UP000000311 | |
| Other | NCBI RefSeq Reference proteome (Camponotus floridanus), v7.5 | NCBI RefSeq | GCF_003227725.1 | |
| Biological sample (Camponotus floridanus) | Trophallactic fluid (see details in Supplementary file 1) | This paper | Supplementary file 1 | Supplementary file 1 |
| Software, algorithm | MaxQuant v1.6.2.10 | MaxQuant | RRID:SCR_014485 | |
| Software, algorithm | Perseus v1.6.15.0 | Perseus | RRID:SCR_015753 | |
| Software, algorithm | R 3.6.1 | R | RRID:SCR_001905 | |
| Software, algorithm | Matlab 2020b | Mathworks | RRID:SCR_001622 | |
| Software, algorithm | R-package MASS 7.3–53 | R Project | RRID:SCR_019125 | |
| Software, algorithm | R-package LME4 | R Project | RRID:SCR_015654 | |
| Software, algorithm | R-package multcomp 1.4–15 | R Project | RRID:SCR_018255 | |
| Software, algorithm | LIMMA-pipeline-proteomics pipeline 3.0.0 | GitHub | 10.5281/zenodo.4050581 | |
| Software, algorithm | sklearn v0.22.1 | Scikit-learn | RRID:SCR_019053 | |
| Software, algorithm | Python 3.7.6 | Python | RRID:SCR_008394 | |
| Software, algorithm | SHapley Additive ExPlanations package v0.37.0 | GitHub | RRID:SCR_021362 | |
| Software, algorithm | OMA Browser | OMA Browser (Martínez et al., 2020 release) | RRID:SCR_011978 | |
| Software, algorithm | Flybase | Flybase | RRID:SCR_006549 | |
| Software, algorithm | STRING v11 | STRING | RRID:SCR_005223 |
Study species
Request a detailed protocolCamponotus floridanus is a common species of carpenter ant in the south-eastern USA, and has already been the focus of previous trophallactic fluid analyses (LeBoeuf et al., 2016; LeBoeuf et al., 2018). They live in dead wood or in man-made structures, often in urban habitats, and forage for honeydew, floral nectar, extra-floral nectar, and arthropod prey. Each colony has a single, singly mated queen (Gadau et al., 1996), and polydomous nest-structures where queenless satellite nests are common. Colonies grow to tens of thousands of workers and produce sexual brood only after multiple years of initial growth. Large established colonies have two morphologically differentiated worker castes, with variably sized small-headed minors focusing on brood care when young and foraging when old, and big-headed majors that engage in nest defense, foraging and food storage (Deyrup, 2017).
Colony and sample identification
Request a detailed protocolThe species was identified based on worker and queen morphology (Deyrup, 2017; Deyrup, 2003; Moreau et al., 2014). In line with previous studies, we use the name C. floridanus with the knowledge that the taxonomy and nomenclature of the C. atriceps complex (to which it belongs) is not fully resolved (Deyrup, 2017).
We collected full young colonies (0–80 workers) and mature colony extracts (30–200 workers) on several Florida Keys islands (Figure 1 and Supplementary file 1) in winter 2019 and 2020. A colony was deemed ‘young’ if the worker population was <100, primarily minors, and the queen was found (meaning both that the species could be clearly identified and that the nest was not a queenless satellite of an established colony), and ‘mature‘ if the colony was larger ( > 1000 individuals visible) and the opened nest contained many large aggressive majors. Young colonies lack majors (Gibson, 1989) and individuals are generally less aggressive. We only collected mature colony samples when we also found larval brood in the opened nest. In our study area, we observed that young colonies are typically found nesting in different material than are mature colonies. Young colonies are often found under stones or in lumps of clay-like mud associated with crab burrows a short distance from the water, whereas the mature colonies were found nesting in large pieces of damp rotting wood.
Laboratory rearing
Request a detailed protocolYoung colonies were brought to the lab and maintained in fluon-coated plastic boxes with a mesh-ventilated lid, at 25 °C with 60 % relative humidity and a 12 hr light/dark cycle. Each colony was provided with one or more glass tube for nesting, 10 % sugar water, and a Bhatkar & Whitcomb diet (Bhatkar and Whitcomb, 1970) and some Drosophila melanogaster. One week prior to proteomic sampling, we substituted the honey-based food with maple syrup-based food to avoid contamination with honeybee proteins (as in LeBoeuf et al., 2016).
Trophallactic fluid collection
Request a detailed protocolField samples of trophallactic fluid were collected within eight hours of ant collection. Of the 20 young colonies and 23 mature colonies, workers collected from two of the mature colonies (L and N) were subdivided into six fragments to assess variation within a single colony (two samples from major workers, two samples from brood-associated workers, and two samples from the remaining minor workers). For all other analyses, only one of these for each colony (referred to as minors1) was used to avoid pseudo-replication. In the laboratory, the trophallactic fluid samples underlying the Field vs. Lab comparison were sampled after six months in the lab. The four colonies used for the single individual analyses had been in the lab for 18 months at the time of trophallactic fluid collection.
Trophallactic fluid was obtained from CO2- or cold-anesthetized workers whose abdomens were gently squeezed to force them to regurgitate the contents of their crops. This method of collection was shown previously to correspond to the fluid shared during the act of adult-adult stomodeal trophallaxis (LeBoeuf et al., 2016). For each colony, at least 30 individuals were sampled to obtain at least 10 µl of raw trophallactic fluid. For many young colonies only smaller samples were possible, because of the low number of workers (Supplementary file 1). Young colony samples were only used for further analysis if at least 2.5 µl of trophallactic fluid were collected. For single individual samples, workers with visibly full abdomens were chosen and the obtained sample volumes ranged from 0.7 µl to 2.2 µl. An individual was classified as forager, when it was seen outside the nest tube in the feeding area of an undisturbed laboratory nest box, and a nurse, when it remained in the nest tube even after the tube was removed from the original laboratory nest and placed into a new one. For colonies from which individual samples were collected, a pooled sample was also taken from individuals that remained after individual sampling. Samples were collected with glass capillaries into 5 µl of 1 x Sigmafast Protease Inhibitor Cocktail (Sigma-Aldrich) with 50 mM Tris pH nine in LoBind eppendorf tubes and were stored –80 C until further analysis. The total proteomics sample number is 73 colony samples of following types: 23 mature colonies with two of them sampled six times, 20 young colonies in the field, 16 young colonies in the laboratory, four laboratory colonies used for single individual sampling; and 40 individual samples: 20 nurses and 20 foragers.
Protein mass spectrometry sample preparation and analysis
Request a detailed protocolSamples were mixed with Laemmli sample buffer and pH was adjusted with 1 M Tris-Cl, pH 7. After reduction with 1 mM DTT for 10 min at 75 °C and alkylation using 5.5 mM iodoacetamide for 10 min at room temperature protein samples were separated on 4–12% gradient gels (ExpressPlus, GeneScript). Each gel lane was cut into small pieces, proteins were in-gel digested with trypsin (Promega) and the resulting peptide mixtures were processed on STAGE tips (Rappsilber et al., 2007; Shevchenko et al., 2006).
LC-MS/MS measurements were performed on a QExactive plus mass spectrometer (Thermo Scientific) coupled to an EasyLC 1000 nanoflow-HPLC. HPLC-column tips (fused silica) with 75 µm inner diameter were self-packed with Reprosil-Pur 120 C18-AQ, 1.9 µm (Dr. Maisch GmbH) to a length of 20 cm. A gradient of A (0.1 % formic acid in water) and B (0.1 % formic acid in 80 % acetonitrile in water) with increasing organic proportion was used for peptide separation (loading of sample with 0 % B; separation ramp: from 5 to 30% B within 85 min). The flow rate was 250 nl/min and for sample application 650 nl/min. The mass spectrometer was operated in the data-dependent mode and switched automatically between MS (max. of 1 × 106 ions) and MS/MS. Each MS scan was followed by a maximum of ten MS/MS scans using normalized collision energy of 25 % and a target value of 1,000. Parent ions with a charge state form z = 1 and unassigned charge states were excluded from fragmentation. The mass range for MS was m/z = 370–1750. The resolution for MS was set to 70,000 and for MS/MS to 17,500. MS parameters were as follows: spray voltage 2.3 kV; no sheath and auxiliary gas flow; ion-transfer tube temperature 250 °C.
The MS raw data files were uploaded into MaxQuant software (Tyanova et al., 2016a), version 1.6.2.10, for peak detection, generation of peak lists of mass error corrected peptides, and for database searches. MaxQuant was set up to search both the UniProt (RRID:SCR_002380, https://www.uniprot.org/) and NCBI (RRID:SCR_003496, https://www.ncbi.nlm.nih.gov/) databases restricted to C. floridanus (UniProt, February 2020 version; NCBI RefSeq, version 7.5), along with common contaminants, such as keratins and enzymes used for digestion. Carbamidomethylcysteine was set as fixed modification and protein amino-terminal acetylation and oxidation of methionine were set as variable modifications. Three missed cleavages were allowed, enzyme specificity was trypsin/P, and the MS/MS tolerance was set to 20 ppm. The average mass precision of identified peptides was in general less than one ppm after recalibration. Peptide lists were further used by MaxQuant to identify and relatively quantify proteins using the following parameters: peptide and protein false discovery rates, based on a forward-reverse database, were set to 0.01, minimum peptide length was set to 7, minimum number of peptides for identification and quantitation of proteins was set to one which must be unique. The ‘match-between-run’ option (0.7 min) was used, which helps improve the protein identifications especially for our single-individual samples. All proteins labelled as contaminants, reverse or only identified by site were excluded and proteins with scores less than 70 were removed. After the filtering, the dataset contained 519 proteins. Quantitative analysis was performed using iBAQ values. Intensity-based absolute quantification (iBAQ) is the quotient of sum of all identified peptides and the number of theoretically observable peptides of a protein (Schwanhäusser et al., 2011).
Statistical analyses
Request a detailed protocolAnalyses of dataset characteristics were performed in Perseus v1.6.15.0 (Tyanova et al., 2016b), R 3.6.1 (R Development Core Team, 2013) and Matlab R2020b (Figures 2 and 3). Differences in protein numbers among the sample types were analyzed with a negative binomial model, using the function nb.glm from the R-package MASS 7.3–53 (Venables and Ripley, 2002). Proteome variability per sample type, as measured by the coefficient of variation of the iBAQ abundance of each protein when present, was analysed with a generalized linear model with gamma distribution and log-link with the R-package LME4 (1.1–26) (Bates et al., 2015). The package multcomp 1.4–15 was used for post-hoc testing for both models. Pearson correlation tests were used to check whether obtained protein number correlates with the sample volume. Because significant correlation was found, all further analyses were done separately for the individual samples that have small volume, and colony samples that have larger volume. Principal component analysis was run in Matlab on raw iBAQ values, for both the individual and the colony datasets.
Metric for self-similarity (S) within and across samples was calculated in Matlab2020b (https://github.com/dradri/variation2021; LeBoeuf, 2021; copy archived at swh:1:rev:4a620922992272317f3cedad3dae6e60871cb282) as follows: pairwise standardized Euclidean distances (dissimilarities, D) were calculated between each pair of samples based on square-root transformed and median subtracted protein abundances; these dissimilarities were averaged for each sample with the other samples within type and with the samples of the other type and divided by the average dissimilarity to all other samples. Thus, self-similarity was calculated as:
To establish the proteins whose abundance differs significantly between sample types, samples were subdivided according to three main comparisons (Figure 1): Young vs. Mature colonies from the field, young colonies in the Field vs. Lab six months later, and individual Nurses vs. Foragers in the lab. In addition, the extent of spatial effects was analyzed for the field-collected Young vs. Mature dataset by dividing the sampling locations to two areas (East vs. West). For the colony data, the differing sample volumes may account for a small proportion of the significant differences in the Young vs. Mature comparison, and to lesser extent in the Field vs. Lab comparison, where sample volume is collinear with the sample type. Our analyses may miss some of the proteins more abundant in the young field collected colonies which have the smallest sample volumes.
Quantitative proteomic comparisons between sample types were performed independently with three different approaches to robustly identify significantly differing proteins: (Szathmáry, 2015) classical frequentist t-tests, (Queller, 1992) linear models with empirical Bayes variance correction, and (Hamilton, 1963) machine-learning paired with modified Shapley values. Our approach is designed to be at the same time conservative and to find most of the differing proteins among our comparisons of the trophallactic fluid. The frequentist t-tests are the most conservative, and they miss some interesting proteins due to their strict model expectations that allow only to use the most common proteins. The empirical Bayes approach to cope with sample variance is a more flexible method that allows use of the entire dataset, finding important hits also among the rarer proteins, although the high amount of missing values, where iBAQ equals zero, makes the model less powerful for these proteins (Kammers et al., 2015). The machine learning approach paired with modified Shapley values, although less well explored in the current proteomics literature, is promising for its ability to find multivariate patterns that the other methods miss, and results in interpretable classification. For each comparison, we report the full results of all three analyses in Supplementary file 2 (significantly differing proteins only) and Supplementary files 3-5 (all results). Our results and discussion sections focus on the proteins that appear significantly different based on two out of three analysis methods (Figure 5).
Classical frequentist analysis
Request a detailed protocolWithin each dataset only proteins present in over 70 % of the samples were analyzed. Out of an original 519 proteins, the final datasets for each comparison contained the following number of proteins: Young vs. Mature, 172; Field vs. Lab, 137; and Nurse vs. Forager, 136. All data were log2 transformed and median-centered, and missing data were imputed by random sampling from normal distribution with 2SD downward shift and 0.3 width for each sample. For colony datasets, we used the permutation-based FDR of 0.05, and for the single individual dataset that contained more borderline-significant proteins, we used a more stable Benjamini-Hochberg FDR with a stricter threshold of 0.01. S0 parameter (similar to fold-change) was set to two for all analyses. All comparisons were run as two-sample t-tests, with the Field vs. Lab as paired.
For the individual dataset, the combined effects of colony identity and behavioral role (Nurse vs. Forager) and their interaction were analyzed with two-way ANOVA, with Benjamini-Hochberg FDR corrections performed in R with the base R 3.6.1 command ‘p.adjust’. Both factors were also analyzed separately with multiple- and two-sample t-tests (for colony identity and behavioral role, respectively). To allow comparison to the other statistical methods, only the simple Nurse vs. Forager analysis without the interaction was used for combining the lists of significantly different protein abundances. Our balanced sampling guarantees the results of this simpler model are robust enough to find the most descriptive proteins for nurse and forager trophallactic fluid, even when the more complex interactive patterns are lost.
Empirical Bayesian analysis
Request a detailed protocolWe implemented LIMMA (Linear Models for Microarray Data), a method for two-group comparison using empirical Bayes methods to moderate the standard errors across proteins (Kammers et al., 2015), on our score-filtered iBAQ proteomic datasets with the LIMMA-pipeline-proteomics pipeline 3.0.0 (http://doi.org/10.5281/zenodo.4050581) developed for R 4.0.2. Data were median-normalized before comparison and all comparisons were run with a log2 fold change cutoff of 2.
Random forest and shap analysis
Request a detailed protocolWe used random forest models (sklearn.ensemble.RandomForestClassifier version 0.22.1 Pedregosa et al., 2011) to classify samples into one of two groups for each comparison. These analyses were performed in Python 3.7.6 in a Jupyter notebook (https://github.com/dradri/variation2021). For each comparison, 10 analyses were performed, each with a different seed. For each seed, the dataset was split into 80 % training set and 20 % test set, and a model was fit, tested and accuracy computed. If accuracy was below 85%, hyper-parameter tuning was performed with GridSearchCV (sklearn 0.22.1), and the model re-fit. A seed and its corresponding model were not retained for further analysis if accuracy could not be improved above 75 %. Accuracies for East vs. West ranged from 33 to 89% and over 20 seeds, only one could be improved above 75 %. The typical parameters: max_depth, 3 or 5; max_features, 'auto'; min_samples_leaf, 3; min_samples_split, 8 or 12; n_estimators, 100 or 500. Samples were classified with out-of-box scores (Supplementary file 4). The average accuracies of classification for comparisons were: Young vs. Mature, 87 %; Nurse vs. Forager, 93 %; Field vs. Lab, 91 %; East vs. West, 58 %.
To understand which proteins contributed to the classification, we used SHAP (SHapley Additive exPlanations, shap package v0.37.0 for Python 3), a game theory tool that explains the output of machine learning models (Lundberg and Lee, 2017). To analyze the importance of each protein in a given comparison (feature importance), we averaged the absolute value of the Shapley values per protein across the data to derive the feature importance. Then for each protein, we averaged the feature importances over each of the 10 seeded models. Proteins that have no impact on the model classification receive a feature importance value of 0. When ranked according to average feature importance, the data had an approximate Pareto distribution with an inflection point typically at feature importance of ~0.15. Thus, because there is no established cutoff for significance in this form of analysis, we chose to include as ‘significant’ in further analyses all proteins with a feature importance of >0.15 (Supplementary file 5).
For random forest predictions, models trained on the classification between young and mature colonies were used to classify the same young colonies after 6 months in the laboratory. Out-of-box scores were averaged over five seeded models.
Orthology, gene ontology, and protein network analyses
Request a detailed protocolBecause little functional work has been done in ants, we analyzed gene ontology terms for the Drosophila orthologs to our genes of interest. Orthologs to C. floridanus trophallactic fluid proteins were determined with OMA (‘Orthologous MAtrix’ Martínez et al., 2020 release Altenhoff et al., 2021). If no ortholog was found within OMA for a given gene, the protein sequence was protein BLASTed against Drosophila melanogaster. In some cases, no ortholog could be found. Annotations were compiled from NCBI RefSeq and UniProt annotations.
GO analysis was performed using both Flybase (Larkin et al., 2021) and STRING v11 (Szklarczyk et al., 2019). STRING was also used for protein-protein interaction and pathway analyses, including KEGG and Reactome (SI Supplementary files 4 and 5). The protein-protein interaction enrichment analysis in STRING used a hypergeometric test with Benjamini-Hochberg corrected FDR. Only 43 out of the 60 most abundant proteins had sufficient annotation for use by STRING while 44 of the 46 differentially abundant proteins had sufficient annotation.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD028568. All other data are made available in this submission in figure supplements, source code and supplementary files.
-
PRIDEID PXD028568. Biomarkers in a socially exchanged fluid reflect colony maturity, behavior and distributed metabolism.
References
-
OMA orthology in 2021: website overhaul, conserved isoforms, ancestral gene order and moreNucleic Acids Research 49:D373–D379.https://doi.org/10.1093/nar/gkaa1007
-
The causes and consequences of genetic caste determination in ants (Hymenoptera: Formicidae)Ecology 11:119–132.
-
Fitting Linear Mixed-Effects Models Using lme4Journal of Statistical Software 67:1–48.https://doi.org/10.18637/jss.v067.i01
-
Artificial Diet for Rearing Various Species of AntsThe Florida Entomologist 53:229.https://doi.org/10.2307/3493193
-
Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translationBiological Reviews of the Cambridge Philosophical Society 93:28–54.https://doi.org/10.1111/brv.12330
-
BookPrinciples of Social EvolutionOxford, UK: Oxford University Press.https://doi.org/10.1093/acprof:oso/9780199231157.001.0001
-
Evolution and function of the insect hexamerinsEuropean Journal of Entomology 96:213–225.
-
Ligands and receptors: Common theme in insect storage protein transportDie Naturwissenschaften 86:468–474.https://doi.org/10.1007/s001140050656
-
Origin and evolution of arthropod hemocyanins and related proteinsJ. Comp. Physiol. B Biochem. Syst. Environ. Physiol 172:95–107.https://doi.org/10.1007/s00360-001-0247-7
-
Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene familyBiological Reviews of the Cambridge Philosophical Society 89:255–269.https://doi.org/10.1111/brv.12052
-
Gene expression patterns associated with queen honey bee longevityMechanisms of Ageing and Development 126:1230–1238.https://doi.org/10.1016/j.mad.2005.07.004
-
Molecular mechanisms of phenotypic plasticity in social insectsCurrent Opinion in Insect Science 13:55–60.https://doi.org/10.1016/j.cois.2015.12.003
-
Collective cognition in animal groupsTrends in Cognitive Sciences 13:36–43.https://doi.org/10.1016/j.tics.2008.10.002
-
Molecular mechanisms of developmentally programmed crinophagy in DrosophilaThe Journal of Cell Biology 217:361–374.https://doi.org/10.1083/jcb.201702145
-
BookAnts of Florida Identification and natural historyBoca Raton, FL: Taylor & Francis Group.
-
BookMechanisms underlying reproductive trade-offs: Costs of reproductionIn: Flatt T, Heyland A, editors. Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs. Oxford, UK: Oxford University Press. pp. 137–152.https://doi.org/10.1093/acprof:oso/9780199568765.003.0011
-
Survival costs of reproduction in DrosophilaExperimental Gerontology 46:369–375.https://doi.org/10.1016/j.exger.2010.10.008
-
Distributed physiology and the molecular basis of social life in eusocial insectsHormones and Behavior 122:104757.https://doi.org/10.1016/j.yhbeh.2020.104757
-
The Evolution of Altruistic BehaviorThe American Naturalist 97:354–356.https://doi.org/10.1086/497114
-
Aging and reproduction in social insects--a mini-reviewGerontology 54:160–167.https://doi.org/10.1159/000122472
-
The plasticity of lifespan in social insectsPhilosophical Transactions of the Royal Society B 376:20190734.https://doi.org/10.1098/rstb.2019.0734
-
Regulation of honey bee division of labor by colony age demographyBehavioral Ecology and Sociobiology 39:147–158.https://doi.org/10.1007/s002650050276
-
Deconstructing the Superorganism: Social Physiology, Groundplans, and SociogenomicsThe Quarterly Review of Biology 85:57–79.https://doi.org/10.1086/650290
-
Detecting significant changes in protein abundanceEuPA Open Proteomics 7:11–19.https://doi.org/10.1016/j.euprot.2015.02.002
-
Cooperation, Conflict, and the Evolution of Queen PheromonesJournal of Chemical Ecology 37:1263–1275.https://doi.org/10.1007/s10886-011-0036-z
-
Comparative transcriptomic analysis of the mechanisms underpinning ageing and fecundity in social insectsPhilosophical Transactions of the Royal Society B 376:20190728.https://doi.org/10.1098/rstb.2019.0728
-
Oxidative stress and senescence in social insects: a significant but inconsistent link?Philosophical Transactions of the Royal Society B 376:20190732.https://doi.org/10.1098/rstb.2019.0732
-
FlyBase: updates to the Drosophila melanogaster knowledge baseNucleic Acids Research 49:D899–D907.https://doi.org/10.1093/nar/gkaa1026
-
Immunity in a variable worldPhilosophical Transactions of the Royal Society B 364:15–26.https://doi.org/10.1098/rstb.2008.0141
-
Softwarevariation2021, version swh:1:rev:4a620922992272317f3cedad3dae6e60871cb282Software Heritage.
-
The molecular and evolutionary genetic implications of being truly social for the social insectsAdvances in Insect Physiology 48:271–292.https://doi.org/10.1016/bs.aiip.2014.12.003
-
Wolbachia-induced paternal defect in Drosophila is likely by interaction with the juvenile hormone pathwaySect Biochemistry and Molecular Biology 49:49–58.https://doi.org/10.1016/j.ibmb.2014.03.014
-
The co‐evolution of longevity and social lifeFunctional Ecology 34:76–87.https://doi.org/10.1111/1365-2435.13445
-
Mitochondrial Bioenergetics and Dynamics in Secretion ProcessesFrontiers in Endocrinology 11:1–18.https://doi.org/10.3389/fendo.2020.00319
-
On the Fly: Recent Progress on Autophagy and Aging in DrosophilaFrontiers in Cell and Developmental Biology 7:1–15.https://doi.org/10.3389/fcell.2019.00140
-
Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae)Myrmecological News 10:4136.
-
Juvenile Hormone and the Physiological Basis of Insect PolymorphismsThe Quarterly Review of Biology 57:109–133.https://doi.org/10.1086/412671
-
The effects of juvenile hormone on Lasius niger reproductionJournal of Insect Physiology 95:1–7.https://doi.org/10.1016/j.jinsphys.2016.09.004
-
Scikit-Learn: Machine Learning in PythonJournal of Machine Learning Research: JMLR 12:2825–2830.
-
A General Model for Kin SelectionEvolution; International Journal of Organic Evolution 46:376.https://doi.org/10.1111/j.1558-5646.1992.tb02045.x
-
SoftwareA Language and Environment for Statistical Computing, version 2.6.2Vienna Austria R Found. Stat. Comput.
-
Advances on human milk hormones and protection against obesityCellular and Molecular Biology 59:89–98.https://doi.org/10.1170/T950
-
Regulation of polyphenic caste differentiation in the termite Reticulitermes flavipes by interaction of intrinsic and extrinsic factorsThe Journal of Experimental Biology 210:4390–4398.https://doi.org/10.1242/jeb.010876
-
Nature versus nurture in social insect caste differentiationTrends in Ecology & Evolution 25:275–282.https://doi.org/10.1016/j.tree.2009.12.001
-
What are biomarkers?Current Opinion in HIV and AIDS 5:463–466.https://doi.org/10.1097/COH.0b013e32833ed177
-
Transcriptomic signatures mirror the lack of the fecundity/longevity trade-off in ant queensMolecular Biology and Evolution 32:3173–3185.https://doi.org/10.1093/molbev/msv186
-
BookThe Social Insects: Their Origin and EvolutionLondon: Wellcome Library.https://doi.org/10.5962/bhl.title.140774
-
Developmental and Physiological Determinants of Caste in Social Hymenoptera: Evolutionary ImplicationsThe American Naturalist 128:13–34.https://doi.org/10.1086/284536
-
Social networks predict the life and death of honey beesNature Communications 12:1–12.https://doi.org/10.1038/s41467-021-21212-5
-
Evolutionary consequences of indirect genetic effectsTrends in Ecology & Evolution 13:64–69.https://doi.org/10.1016/S0169-5347(97)01233-0
Article and author information
Author details
Funding
Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (PR00P3_179776)
- Adria C LeBoeuf
Bundesbehörden der Schweizerischen Eidgenossenschaft (2020.0228)
- Sanja M Hakala
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Joanne Reiter for help with field work, Guillaume Kuhn for help with ant maintenance in the laboratory, and members of the Social Fluids lab and the Review Commons reviewers for their useful comments on the manuscript.
Copyright
© 2021, Hakala et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,536
- views
-
- 409
- downloads
-
- 14
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Developmental Biology
The spatial and temporal linear expression of Hox genes establishes a regional Hox code, which is crucial for the antero-posterior (A-P) patterning, segmentation, and neuronal circuit development of the hindbrain. RNF220, an E3 ubiquitin ligase, is widely involved in neural development via targeting of multiple substrates. Here, we found that the expression of Hox genes in the pons was markedly up-regulated at the late developmental stage (post-embryonic day E15.5) in Rnf220-/- and Rnf220+/- mouse embryos. Single-nucleus RNA sequencing (RNA-seq) analysis revealed different Hox de-repression profiles in different groups of neurons, including the pontine nuclei (PN). The Hox pattern was disrupted and the neural circuits were affected in the PN of Rnf220+/- mice. We showed that this phenomenon was mediated by WDR5, a key component of the TrxG complex, which can be polyubiquitinated and degraded by RNF220. Intrauterine injection of WDR5 inhibitor (WDR5-IN-4) and genetic ablation of Wdr5 in Rnf220+/- mice largely recovered the de-repressed Hox expression pattern in the hindbrain. In P19 embryonal carcinoma cells, the retinoic acid-induced Hox expression was further stimulated by Rnf220 knockdown, which can also be rescued by Wdr5 knockdown. In short, our data suggest a new role of RNF220/WDR5 in Hox pattern maintenance and pons development in mice.
-
- Developmental Biology
- Ecology
Organisms require dietary macronutrients in specific ratios to maximize performance, and variation in macronutrient requirements plays a central role in niche determination. Although it is well recognized that development and body size can have strong and predictable effects on many aspects of organismal function, we lack a predictive understanding of ontogenetic or scaling effects on macronutrient intake. We determined protein and carbohydrate intake throughout development on lab populations of locusts and compared to late instars of field populations. Self-selected protein:carbohydrate targets declined dramatically through ontogeny, due primarily to declines in mass-specific protein consumption rates which were highly correlated with declines in specific growth rates. Lab results for protein consumption rates partly matched results from field-collected locusts. However, field locusts consumed nearly double the carbohydrate, likely due to higher activity and metabolic rates. Combining our results with the available data for animals, both across species and during ontogeny, protein consumption scaled predictably and hypometrically, demonstrating a new scaling rule key for understanding nutritional ecology.






