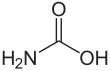
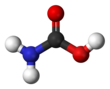
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid,[2] is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases.[3] The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.[4]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Carbamic acid[1] | |||
| Other names
Aminomethanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
| MeSH | Carbamic+acid | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NH3CO2 | |||
| Molar mass | 61.040 g·mol−1 | ||
| Related compounds | |||
Related compounds
|
Formamide Dithiocarbamate Carbonic acid Urea Ethyl carbamate Sulfamic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Carbamic acid could be seen as both an amine and carboxylic acid, and therefore an amino acid;[3] however, the attachment of the carboxyl group –COOH directly to the nitrogen atom (without any intermediate carbon chain) makes it behave very differently from the amino acids with intermediate carbon chain. (Glycine NH2CH2COOH is generally considered to be the simplest amino acid.) The hydroxyl group –OH attached to the carbon also excludes it from the amide class.
The term "carbamic acid" is also used generically for any compounds of the form RR′NCOOH, where R and R′ are organic groups or hydrogen.[5]
Deprotonation of a carbamic acid yields a carbamate anion RR′NCOO−, the salts of which can be relatively stable. Carbamate is also a term used for esters of carbamic acids, such as methyl carbamate H2N−C(=O)−OCH3. The carbamoyl functional group RR′N–C(=O)– (often denoted by Cbm) is the carbamic acid molecule minus the OH part of the carboxyl.
Structure
editCarbamic acid is a planar molecule.[3]
The H2N− group of carbamic acid, unlike that of most amines, cannot be protonated to an ammonium group H3N+−. The zwitterionic form H3N+−COO− is very unstable and promptly decomposes into ammonia and carbon dioxide,[6] yet there is a report of its detection in ices irradiated with high-energy protons.[3]
Derivatives
editCarbamic acid is formally the parent compound of several important families of organic compounds:
-
carbamic acids
-
carbamate anions
-
carbamate esters
Carbamic acids
editMany substituted carbamic acids (RHNCOOH or RR′NCOOH), can be readily synthesized by bubbling carbon dioxide through solutions of the corresponding amine (RNH2 or RR′NH, respectively) in an appropriate solvent, such as DMSO or supercritical carbon dioxide.[5] These carbamic acids are generally unstable at room temperature, reverting to the parent amine and carbon dioxide.[7]
Carbamate esters
editUnlike carbamic acids, carbamate esters are generally stable at room temperature as a higher state. They are prepared by reaction of carbamoyl chlorides with alcohols, the addition of alcohols to isocyanates, and the reaction of carbonate esters with ammonia.[8] Methyl carbamate and ethyl carbamate are among the simplest examples and have historically been used in the textile industry, both are now suspected carcinogens. Benzyl carbamate is also known.
Occurrence in nature
editThe enzyme class carbamate kinase, involved in several metabolic pathways of living organisms, catalyzes the formation of carbamoyl phosphate H2N−C(=O)−O−PO2−3:
An important example of an enzyme with this activity is carbamoyl phosphate synthetase, e.g. carbamoyl phosphate synthetase I carrying out the first step of the urea cycle in order to dispose of waste ammonia.
One hemoglobin molecule can carry four molecules of carbon dioxide to the lungs as carbamate groups formed by reaction of CO2 with four terminal amine groups of the deoxy form. The resulting compound is called carbaminohaemoglobin.
Uses
editIndustrial
editCarbamic acid is an intermediate in the industrial production of urea, which involves the reaction of carbon dioxide and ammonia.[9]
- CO2 + NH3 → H2NCOOH
- H2NCOOH + NH3 → CO(NH2)2 + H2O
Medical
editSome carbamate esters have use as muscle relaxants, including Emylcamate, Phenprobamate, Styramate and other members of ATC code M03BA. These bind to the barbiturate site of the GABAA receptor.[10]
Insecticides
editSeveral carbamic acid based insecticides have been developed; for example aldicarb, carbaryl, carbofuran.[11]
Chemical synthesis
editAn amine functional group −NH2 can be protected from unwanted reactions by being formed as carbamate ester residue –NHC(=O)–OR. Hydrolysis of the ester bond then produces a carbamic acid –NHC(=O)OH, which then loses carbon dioxide yielding the desired amine.
References
edit- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 778. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ "PubChem Compound Summary for CID 277, Carbamic acid". National Center for Biotechnology Information. 2020. Retrieved October 10, 2020.
- ^ a b c d R. K. Khanna and M. H. Moore (1999): "Carbamic acid: molecular structure and IR spectra". Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, volume 55, issue 5, pages 961-967. doi:10.1016/S1386-1425(98)00228-5 PMID 10347902Bibcode:1999AcSpA..55..961K
- ^ J. B. Bossa, P. Theulé, F. Duvernay, F. Borget and T. Chiavassa (2008): "Carbamic acid and carbamate formation in NH3:CO2 ices – UV irradiation versus thermal processes". Astronomy and Astrophysics, volume 492, issue 3, pages 719-724. doi:10.1051/0004-6361:200810536
- ^ a b Z. J. Dijkstra, A. R. Doornbos, H. Weyten, J. M. Ernsting, C. J. Elsevier, and J. T. F. Keurentjes (2007): "Formation of carbamic acid in organic solvents and in supercritical carbon dioxide". Journal of Supercritical Fluids, volume 41, issue 1, pages 109-114. doi:10.1016/j.supflu.2006.08.012
- ^ Y.-J. Chen, M. Nuevo, J.-M. Hsieh, T.-S. Yih, W.-H. Sun, W.-H. Ip, H.-S. Fung, S.-Y. Chiang, Y.-Y. Lee, J.-M. Chen and C.-Y. R. Wu (2007): "Carbamic acid produced by the UV/EUV irradiation of interstellar ice analogs". Astronomy and Astrophysics, volume 464, issue 1, pages 253-257. doi:10.1051/0004-6361:20066631
- ^ Lemke, Thomas L. (2003). Review of Organic Functional Groups: Introduction to Medicinal Organic Chemistry. Philadelphia, PA: Lippincott, Williams & Wilkins. p. 63. ISBN 978-0-7817-4381-5.
- ^ Jäger, Peter; Rentzea, Costin N.; Kieczka, Heinz (2000). "Carbamates and Carbamoyl Chlorides". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a05_051. ISBN 3527306730.
- ^ Meessen, J. H.; Petersen, H. "Urea". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_333. ISBN 978-3527306732.
- ^ Block, John H.; Beale, John M., eds. (2004). "Central Nervous System Depressant". Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry. Philadelphia, PA: Lippincott, Williams & Wilkins. p. 495. ISBN 978-0-7817-3481-3.
- ^ Risher, John F.; Mink, Franklin L.; Stara, Jerry F. (1987). "The Toxicologic Effects of the Carbamate Insecticide Aldicarb in Mammals: A Review". Environmental Health Perspectives. 72: 267–281. doi:10.2307/3430304. JSTOR 3430304. PMC 1474664. PMID 3304999.