An estrogen conjugate is a conjugate of an endogenous estrogen. They occur naturally in the body as metabolites of estrogens and can be reconverted back into estrogens. They serve as a circulating reservoir for estrogen, particularly in the case of orally administered pharmaceutical estradiol. Estrogen conjugates include sulfate and/or glucuronide conjugates of estradiol, estrone, and estriol:

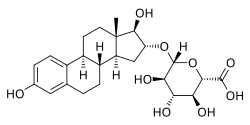
- Sulfated:
- Estrone 3-sulfate (E1S)
- Estradiol 3-sulfate (E2S, E2-3S) and estradiol 17
β -sulfate (E2-17S) - Estriol 3-sulfate (E3S)
- Estradiol 3,17
β -disulfate (E2DS)
- Glucuronidated:
- Estrone 3-glucuronide (E1-G)
- Estradiol 3-glucuronide (E2-3G) and estradiol 17
β -glucuronide (E2-17G) - Estriol 3-glucuronide (E3-3G), estriol 16
α -glucuronide (E3-16G)
- Mixed:
- Estradiol 3-glucuronide 17
β -sulfate (E2-3G-17β S) - Estradiol 3-sulfate 17
β -glucuronide (E2-3S-17β G) - Estriol 3-sulfate 16
α -glucuronide (E3-3S-16G)
- Estradiol 3-glucuronide 17
Estrogen conjugates are conjugated at the C3, C16
Estrogen conjugates have been used as pharmaceutical estrogens, as in estrone sulfate as estropipate (piperazine estrone sulfate) and in conjugated estrogens (Premarin) and conjugated estriol (Progynon, Emmenin).
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ER |
ER | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17 |
E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17 |
E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17 |
? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17 |
19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17 |
2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17 |
?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17 |
0 | ? | ? | ||
| Estradiol stearate | Estradiol 17 |
0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17 |
100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro | ||||||
| Ligand | Other names | Relative binding affinities (RBA, %)a | Absolute binding affinities (Ki, nM)a | Action | ||
|---|---|---|---|---|---|---|
| ER |
ER |
ER |
ER | |||
| Estradiol | E2; 17 |
100 | 100 | 0.115 (0.04–0.24) | 0.15 (0.10–2.08) | Estrogen |
| Estrone | E1; 17-Ketoestradiol | 16.39 (0.7–60) | 6.5 (1.36–52) | 0.445 (0.3–1.01) | 1.75 (0.35–9.24) | Estrogen |
| Estriol | E3; 16 |
12.65 (4.03–56) | 26 (14.0–44.6) | 0.45 (0.35–1.4) | 0.7 (0.63–0.7) | Estrogen |
| Estetrol | E4; 15 |
4.0 | 3.0 | 4.9 | 19 | Estrogen |
| Alfatradiol | 17 |
20.5 (7–80.1) | 8.195 (2–42) | 0.2–0.52 | 0.43–1.2 | Metabolite |
| 16-Epiestriol | 16 |
7.795 (4.94–63) | 50 | ? | ? | Metabolite |
| 17-Epiestriol | 16 |
55.45 (29–103) | 79–80 | ? | ? | Metabolite |
| 16,17-Epiestriol | 16 |
1.0 | 13 | ? | ? | Metabolite |
| 2-Hydroxyestradiol | 2-OH-E2 | 22 (7–81) | 11–35 | 2.5 | 1.3 | Metabolite |
| 2-Methoxyestradiol | 2-MeO-E2 | 0.0027–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Hydroxyestradiol | 4-OH-E2 | 13 (8–70) | 7–56 | 1.0 | 1.9 | Metabolite |
| 4-Methoxyestradiol | 4-MeO-E2 | 2.0 | 1.0 | ? | ? | Metabolite |
| 2-Hydroxyestrone | 2-OH-E1 | 2.0–4.0 | 0.2–0.4 | ? | ? | Metabolite |
| 2-Methoxyestrone | 2-MeO-E1 | <0.001–<1 | <1 | ? | ? | Metabolite |
| 4-Hydroxyestrone | 4-OH-E1 | 1.0–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestrone | 4-MeO-E1 | <1 | <1 | ? | ? | Metabolite |
| 16 |
16 |
2.0–6.5 | 35 | ? | ? | Metabolite |
| 2-Hydroxyestriol | 2-OH-E3 | 2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestriol | 4-MeO-E3 | 1.0 | 1.0 | ? | ? | Metabolite |
| Estradiol sulfate | E2S; Estradiol 3-sulfate | <1 | <1 | ? | ? | Metabolite |
| Estradiol disulfate | Estradiol 3,17 |
0.0004 | ? | ? | ? | Metabolite |
| Estradiol 3-glucuronide | E2-3G | 0.0079 | ? | ? | ? | Metabolite |
| Estradiol 17 |
E2-17G | 0.0015 | ? | ? | ? | Metabolite |
| Estradiol 3-gluc. 17 |
E2-3G-17S | 0.0001 | ? | ? | ? | Metabolite |
| Estrone sulfate | E1S; Estrone 3-sulfate | <1 | <1 | >10 | >10 | Metabolite |
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | ? | ? | ? | Estrogen |
| Estradiol 17 |
E2-17B | 11.3 | 32.6 | ? | ? | Estrogen |
| Estrone methyl ether | Estrone 3-methyl ether | 0.145 | ? | ? | ? | Estrogen |
| ent-Estradiol | 1-Estradiol | 1.31–12.34 | 9.44–80.07 | ? | ? | Estrogen |
| Equilin | 7-Dehydroestrone | 13 (4.0–28.9) | 13.0–49 | 0.79 | 0.36 | Estrogen |
| Equilenin | 6,8-Didehydroestrone | 2.0–15 | 7.0–20 | 0.64 | 0.62 | Estrogen |
| 17 |
7-Dehydro-17 |
7.9–113 | 7.9–108 | 0.09 | 0.17 | Estrogen |
| 17 |
7-Dehydro-17 |
18.6 (18–41) | 14–32 | 0.24 | 0.57 | Estrogen |
| 17 |
6,8-Didehydro-17 |
35–68 | 90–100 | 0.15 | 0.20 | Estrogen |
| 17 |
6,8-Didehydro-17 |
20 | 49 | 0.50 | 0.37 | Estrogen |
| 8,9-Dehydro-17 |
68 | 72 | 0.15 | 0.25 | Estrogen | |
| 8,9-Dehydroestrone | 19 | 32 | 0.52 | 0.57 | Estrogen | |
| Ethinylestradiol | EE; 17 |
120.9 (68.8–480) | 44.4 (2.0–144) | 0.02–0.05 | 0.29–0.81 | Estrogen |
| Mestranol | EE 3-methyl ether | ? | 2.5 | ? | ? | Estrogen |
| Moxestrol | RU-2858; 11 |
35–43 | 5–20 | 0.5 | 2.6 | Estrogen |
| Methylestradiol | 17 |
70 | 44 | ? | ? | Estrogen |
| Diethylstilbestrol | DES; Stilbestrol | 129.5 (89.1–468) | 219.63 (61.2–295) | 0.04 | 0.05 | Estrogen |
| Hexestrol | Dihydrodiethylstilbestrol | 153.6 (31–302) | 60–234 | 0.06 | 0.06 | Estrogen |
| Dienestrol | Dehydrostilbestrol | 37 (20.4–223) | 56–404 | 0.05 | 0.03 | Estrogen |
| Benzestrol (B2) | – | 114 | ? | ? | ? | Estrogen |
| Chlorotrianisene | TACE | 1.74 | ? | 15.30 | ? | Estrogen |
| Triphenylethylene | TPE | 0.074 | ? | ? | ? | Estrogen |
| Triphenylbromoethylene | TPBE | 2.69 | ? | ? | ? | Estrogen |
| Tamoxifen | ICI-46,474 | 3 (0.1–47) | 3.33 (0.28–6) | 3.4–9.69 | 2.5 | SERM |
| Afimoxifene | 4-Hydroxytamoxifen; 4-OHT | 100.1 (1.7–257) | 10 (0.98–339) | 2.3 (0.1–3.61) | 0.04–4.8 | SERM |
| Toremifene | 4-Chlorotamoxifen; 4-CT | ? | ? | 7.14–20.3 | 15.4 | SERM |
| Clomifene | MRL-41 | 25 (19.2–37.2) | 12 | 0.9 | 1.2 | SERM |
| Cyclofenil | F-6066; Sexovid | 151–152 | 243 | ? | ? | SERM |
| Nafoxidine | U-11,000A | 30.9–44 | 16 | 0.3 | 0.8 | SERM |
| Raloxifene | – | 41.2 (7.8–69) | 5.34 (0.54–16) | 0.188–0.52 | 20.2 | SERM |
| Arzoxifene | LY-353,381 | ? | ? | 0.179 | ? | SERM |
| Lasofoxifene | CP-336,156 | 10.2–166 | 19.0 | 0.229 | ? | SERM |
| Ormeloxifene | Centchroman | ? | ? | 0.313 | ? | SERM |
| Levormeloxifene | 6720-CDRI; NNC-460,020 | 1.55 | 1.88 | ? | ? | SERM |
| Ospemifene | Deaminohydroxytoremifene | 0.82–2.63 | 0.59–1.22 | ? | ? | SERM |
| Bazedoxifene | – | ? | ? | 0.053 | ? | SERM |
| Etacstil | GW-5638 | 4.30 | 11.5 | ? | ? | SERM |
| ICI-164,384 | – | 63.5 (3.70–97.7) | 166 | 0.2 | 0.08 | Antiestrogen |
| Fulvestrant | ICI-182,780 | 43.5 (9.4–325) | 21.65 (2.05–40.5) | 0.42 | 1.3 | Antiestrogen |
| Propylpyrazoletriol | PPT | 49 (10.0–89.1) | 0.12 | 0.40 | 92.8 | ER |
| 16 |
16 |
14.6–57 | 0.089 | 0.27 | 131 | ER |
| 16 |
16 |
30.2 | 2.30 | ? | ? | ER |
| Methylpiperidinopyrazole | MPP | 11 | 0.05 | ? | ? | ER |
| Diarylpropionitrile | DPN | 0.12–0.25 | 6.6–18 | 32.4 | 1.7 | ER |
| 8 |
8 |
0.35 | 22.0–83 | 12.9 | 0.50 | ER |
| Prinaberel | ERB-041; WAY-202,041 | 0.27 | 67–72 | ? | ? | ER |
| ERB-196 | WAY-202,196 | ? | 180 | ? | ? | ER |
| Erteberel | SERBA-1; LY-500,307 | ? | ? | 2.68 | 0.19 | ER |
| SERBA-2 | – | ? | ? | 14.5 | 1.54 | ER |
| Coumestrol | – | 9.225 (0.0117–94) | 64.125 (0.41–185) | 0.14–80.0 | 0.07–27.0 | Xenoestrogen |
| Genistein | – | 0.445 (0.0012–16) | 33.42 (0.86–87) | 2.6–126 | 0.3–12.8 | Xenoestrogen |
| Equol | – | 0.2–0.287 | 0.85 (0.10–2.85) | ? | ? | Xenoestrogen |
| Daidzein | – | 0.07 (0.0018–9.3) | 0.7865 (0.04–17.1) | 2.0 | 85.3 | Xenoestrogen |
| Biochanin A | – | 0.04 (0.022–0.15) | 0.6225 (0.010–1.2) | 174 | 8.9 | Xenoestrogen |
| Kaempferol | – | 0.07 (0.029–0.10) | 2.2 (0.002–3.00) | ? | ? | Xenoestrogen |
| Naringenin | – | 0.0054 (<0.001–0.01) | 0.15 (0.11–0.33) | ? | ? | Xenoestrogen |
| 8-Prenylnaringenin | 8-PN | 4.4 | ? | ? | ? | Xenoestrogen |
| Quercetin | – | <0.001–0.01 | 0.002–0.040 | ? | ? | Xenoestrogen |
| Ipriflavone | – | <0.01 | <0.01 | ? | ? | Xenoestrogen |
| Miroestrol | – | 0.39 | ? | ? | ? | Xenoestrogen |
| Deoxymiroestrol | – | 2.0 | ? | ? | ? | Xenoestrogen |
| – | <0.001–0.0875 | <0.001–0.016 | ? | ? | Xenoestrogen | |
| Resveratrol | – | <0.001–0.0032 | ? | ? | ? | Xenoestrogen |
| – | 48 (13–52.5) | ? | ? | ? | Xenoestrogen | |
| – | 0.6 (0.032–13) | ? | ? | ? | Xenoestrogen | |
| Zeranol | 48–111 | ? | ? | ? | Xenoestrogen | |
| Taleranol | 16 (13–17.8) | 14 | 0.8 | 0.9 | Xenoestrogen | |
| Zearalenone | ZEN | 7.68 (2.04–28) | 9.45 (2.43–31.5) | ? | ? | Xenoestrogen |
| Zearalanone | ZAN | 0.51 | ? | ? | ? | Xenoestrogen |
| Bisphenol A | BPA | 0.0315 (0.008–1.0) | 0.135 (0.002–4.23) | 195 | 35 | Xenoestrogen |
| Endosulfan | EDS | <0.001–<0.01 | <0.01 | ? | ? | Xenoestrogen |
| Kepone | Chlordecone | 0.0069–0.2 | ? | ? | ? | Xenoestrogen |
| o,p'-DDT | – | 0.0073–0.4 | ? | ? | ? | Xenoestrogen |
| p,p'-DDT | – | 0.03 | ? | ? | ? | Xenoestrogen |
| Methoxychlor | p,p'-Dimethoxy-DDT | 0.01 (<0.001–0.02) | 0.01–0.13 | ? | ? | Xenoestrogen |
| HPTE | Hydroxychlor; p,p'-OH-DDT | 1.2–1.7 | ? | ? | ? | Xenoestrogen |
| Testosterone | T; 4-Androstenolone | <0.0001–<0.01 | <0.002–0.040 | >5000 | >5000 | Androgen |
| Dihydrotestosterone | DHT; 5 |
0.01 (<0.001–0.05) | 0.0059–0.17 | 221–>5000 | 73–1688 | Androgen |
| Nandrolone | 19-Nortestosterone; 19-NT | 0.01 | 0.23 | 765 | 53 | Androgen |
| Dehydroepiandrosterone | DHEA; Prasterone | 0.038 (<0.001–0.04) | 0.019–0.07 | 245–1053 | 163–515 | Androgen |
| 5-Androstenediol | A5; Androstenediol | 6 | 17 | 3.6 | 0.9 | Androgen |
| 4-Androstenediol | – | 0.5 | 0.6 | 23 | 19 | Androgen |
| 4-Androstenedione | A4; Androstenedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| 3 |
3 |
0.07 | 0.3 | 260 | 48 | Androgen |
| 3 |
3 |
3 | 7 | 6 | 2 | Androgen |
| Androstanedione | 5 |
<0.01 | <0.01 | >10000 | >10000 | Androgen |
| Etiocholanedione | 5 |
<0.01 | <0.01 | >10000 | >10000 | Androgen |
| Methyltestosterone | 17 |
<0.0001 | ? | ? | ? | Androgen |
| Ethinyl-3 |
17 |
4.0 | <0.07 | ? | ? | Estrogen |
| Ethinyl-3 |
17 |
50 | 5.6 | ? | ? | Estrogen |
| Progesterone | P4; 4-Pregnenedione | <0.001–0.6 | <0.001–0.010 | ? | ? | Progestogen |
| Norethisterone | NET; 17 |
0.085 (0.0015–<0.1) | 0.1 (0.01–0.3) | 152 | 1084 | Progestogen |
| Norethynodrel | 5(10)-Norethisterone | 0.5 (0.3–0.7) | <0.1–0.22 | 14 | 53 | Progestogen |
| Tibolone | 7 |
0.5 (0.45–2.0) | 0.2–0.076 | ? | ? | Progestogen |
| 7 |
0.069–<0.1 | 0.027–<0.1 | ? | ? | Progestogen | |
| 3 |
– | 2.5 (1.06–5.0) | 0.6–0.8 | ? | ? | Progestogen |
| 3 |
– | 1.6 (0.75–1.9) | 0.070–0.1 | ? | ? | Progestogen |
| Footnotes: a = (1) Binding affinity values are of the format "median (range)" (# (#–#)), "range" (#–#), or "value" (#) depending on the values available. The full sets of values within the ranges can be found in the Wiki code. (2) Binding affinities were determined via displacement studies in a variety of in-vitro systems with labeled estradiol and human ER | ||||||