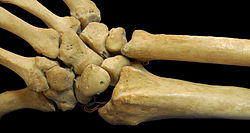
Short bones are designated as those bones that are more or less equal in length, width, and thickness. They include the tarsals in the ankle and the carpals in the wrist. They are one of five types of bones: short, long, flat, irregular and sesamoid. Most short bones are named according to their shape as they exhibit a variety of complex morphological features (They can be cuboid, lenticular, trapezoidal, etc.)[1][2]
| Short bone | |
|---|---|
 | |
 | |
| Details | |
| Identifiers | |
| Latin | os breve |
| TA98 | A02.0.00.012 |
| TA2 | 370 |
| FMA | 7475 |
| Anatomical terms of bone | |
Some authors state that short bones are only located in the carpals and tarsals.[3] The metacarpals, metatarsals and phalanges are considered long bones as they have a shaft (tubular diaphysis), but since they're smaller than typical long bones, they're called “miniature, small or short" long bones.[1][4] Nevertheless, others consider the patellae and other sesamoid bones, the vertebral bodies, the bones of the skull base and even the phalanges to be short bones.[2][5]
Structure
editThe carpus and tarsus consist of cancellous tissue covered by a thin crust of compact substance.[5] Short bones are specialized to provide support in areas of the skeleton that are subjected to high forces or need to be very compact and where strength and stability are more important than range of motion.[1] Short bones are characterized by their multiple articular surfaces and their tendency to form movable joints with adjacent bones. The articular surfaces of short bones are covered with hyaline cartilage, similar to long bones. The outer surface of the bone, except for the articular surfaces, is covered by the periosteum.[6] Short bones have no clear diaphysis (bone shaft) and metaphysis and have poor vascular supply.[1][2]
Development
editBoth short and long bones undergo endochondral ossification during development. In this process, bone is formed from an initial cartilaginous model and this model is then gradually replaced by bone. Despite sharing a common cellular origin, short and long bones have different structural features.[7]
Long bones have epiphyseal growth plates, where chondrocytes, stacked on top of each other, form longitudinal columns that are responsible for longitudinal growth of the bone. Long bones also have secondary ossification centers, in which cell columns are arranged in a radial pattern from the center like spokes on a wheel and cartilage-to-bone replacement starts in the center and extends centrifugally outwards.[2][8]
Contrary to long bones, the carpals and tarsals typically lack epiphyseal growth plates, hence lacking longitudinal growth and they undergo ossification radially, similar to secondary ossification centers in long bones.[9][10][11] As a result, short bones usually develop from a single ossification nucleus, while long bones usually develop from multiple ossification nuclei.[12]
Clinical significance
editShort bones are more prone to nonunion, malunion or osteonecrosis in case of fractures due to their tenuous vascular supply leading to lower healing potential. In contrast, the mid-diaphysis of the femur has a robust vascular supply from the surrounding muscle, and typically heals relatively quickly and reliably.[1][13] This risk of diminished healing of short bone fractures increases in diabetic patients, probably due to diabetic peripheral neuropathy and microvascular dysfunction.[14]
References
edit- ^ a b c d e Bilo, Rob A. C.; Loeve, Arjo A. J.; Robben, Simon G. F.; van Rijn, Rick R. (2023). "General Aspects of Fractures in Children". Forensic Aspects of Paediatric Fractures: Differentiating Accidental Trauma from Child Abuse. Springer International Publishing. pp. 23–43. doi:10.1007/978-3-031-12041-1_2. ISBN 978-3-031-12041-1.
- ^ a b c d De Buffrénil, V; De Ricqlès, A; Zylberberg, L; Padian, K (2021). Vertebrate skeletal histology and paleohistology (1st ed.). Boca Raton London New York: CRC Press, Taylor & Francis Group. doi:10.1201/9781351189590. ISBN 978-0815392880. S2CID 236406115.
- ^ Peate, Ian (2 January 2018). "Anatomy and physiology, 5. The musculoskeletal system". British Journal of Healthcare Assistants. 12 (1): 6–9. doi:10.12968/bjha.2018.12.1.6.
- ^ Singh, V (12 May 2020). General Anatomy with Systemic Anatomy, Radiological Anatomy, Medical Genetics (3rd ed.). Elsevier Health Sciences. p. 69. ISBN 978-81-312-6244-3.
- ^ a b Gray, Henry; Lewis, Warren Harmon (1918). Anatomy of the human body (20th ed.). Philadelphia : Lea & Febiger.
- ^ Ross, Michael H.; Pawlina, Wojciech (2016). Histology: a text and atlas ; with correlated cell and molecular biology (Seventh ed.). Philadelphia: Wolters Kluwer Health. ISBN 978-1451187427.
- ^ Cowan, PT; Kahai, P (2023), "Anatomy, Bones", StatPearls, Treasure Island, Florida (FL): StatPearls Publishing, PMID 30725884
- ^ Standring, S (2016). Gray's anatomy: the anatomical basis of clinical practice ; [get full access and more at ExpertConsult.com] (41. ed.). Philadelphia, Pa.: Elsevier. ISBN 978-0702052309.
- ^ Kjosness, KM; Hines, JE; Lovejoy, CO; Reno, PL (November 2014). "The pisiform growth plate is lost in humans and supports a role for Hox in growth plate formation". Journal of Anatomy. 225 (5): 527–38. doi:10.1111/joa.12235. PMC 4292754. PMID 25279687.
- ^ Reno, Philip L.; Mcburney, Denise L.; Lovejoy, C. Owen; Horton, Walter E. (January 2006). "Ossification of the mouse metatarsal: Differentiation and proliferation in the presence/absence of a defined growth plate". The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 288A (1): 104–118. doi:10.1002/ar.a.20268. ISSN 1552-4884. PMID 16342215.
- ^ Francillon-Vieillot, H.; de Buffrénil, V.; Castanet, J.; Géraudie, J.; Meunier, F.J.; Sire, J. Y.; Zylberberg, L.; de Ricqlès, A. (22 March 2013). "Microstructure and Mineralization of Vertebrate Skeletal Tissues". Short Courses in Geology: 175–234. doi:10.1029/SC005p0175. ISBN 9781118667279.
- ^ Putz, R; Boszczyk, B; Milz, S (October 2019). "How the Ends of Bones Evolve and What They Do: The Anatomical and Biomechanical Perspective". Seminars in Musculoskeletal Radiology. 23 (5): 467–476. doi:10.1055/s-0039-1693977. PMID 31556082. S2CID 203437965.
- ^ Nicksic, PJ; Donnelly, DT; Verma, N; Setiz, AJ; Shoffstall, AJ; Ludwig, KA; Dingle, AM; Poore, SO (2022). "Electrical Stimulation of Acute Fractures: A Narrative Review of Stimulation Protocols and Device Specifications". Frontiers in Bioengineering and Biotechnology. 10: 879187. doi:10.3389/fbioe.2022.879187. PMC 9201474. PMID 35721861. This article incorporates text available under the CC BY 4.0 license.
- ^ Ding, Zi‐chuan; Zeng, Wei‐nan; Rong, Xiao; Liang, Zhi‐min; Zhou, Zong‐ke (July 2020). "Do patients with diabetes have an increased risk of impaired fracture healing? A systematic review and meta‐analysis". ANZ Journal of Surgery. 90 (7–8): 1259–1264. doi:10.1111/ans.15878. ISSN 1445-1433. PMID 32255244. S2CID 215408852.