Beta helix: Difference between revisions
Citation bot (talk | contribs) Add: pmid. | Use this bot. Report bugs. | Suggested by Abductive | Category:Protein folds | #UCB_Category 28/30 |
m Bot: link syntax |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
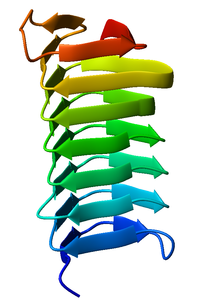
[[Image:1m8n_Choristoneura_fumiferana.png|thumb|right|200px|Monomeric, left-handed |
[[Image:1m8n_Choristoneura_fumiferana.png|thumb|right|200px|Monomeric, left-handed |
||
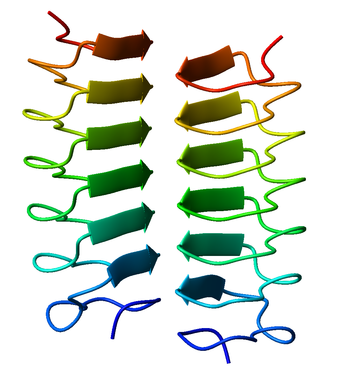
[[Image:1ezg_Tenebrio_molitor.png|thumb|350px|Dimeric, right-handed |
[[Image:1ezg_Tenebrio_molitor.png|thumb|350px|Dimeric, right-handed |
||
A '''beta helix''' is a [[Protein tandem repeats|tandem protein repeat]] structure formed by the association of parallel [[beta sheet |
A '''beta helix''' is a [[Protein tandem repeats|tandem protein repeat]] structure formed by the association of parallel [[beta sheet]] in a helical pattern with either two<ref>{{cite web|title=CATH database - folds and homologous superfamilies within the beta 2-solenoid architecture.|url=http://www.cathdb.info/cathnode/2.150|website=[[CATH database]]}}</ref> or three<ref>{{cite web|title=CATH database - folds and homologous superfamilies within the beta 3-solenoid architecture.|url=http://www.cathdb.info/cathnode/2.160|archive-url=https://web.archive.org/web/20110726124637/http://www.cathdb.info/cathnode/2.160|archive-date=26 July 2011|url-status=dead|website=[[CATH database]]}}</ref> faces. The beta helix is a type of [[solenoid protein domain]]. The structure is stabilized by inter-strand [[hydrogen bond]]s, [[protein-protein interaction]]s, and sometimes bound metal [[ion]]s. Both left- and right-handed beta helices have been identified. These structures are distinct from [[jelly-roll fold]]s, a different protein structure sometimes known as a "double-stranded beta helix".<ref name="aik_2012">{{cite journal |last1=Aik |first1=WeiShen |last2=McDonough |first2=Michael A |last3=Thalhammer |first3=Armin |last4=Chowdhury |first4=Rasheduzzaman |last5=Schofield |first5=Christopher J |title=Role of the jelly-roll fold in substrate binding by 2-oxoglutarate oxygenases |journal=Current Opinion in Structural Biology |date=December 2012 |volume=22 |issue=6 |pages=691–700 |doi=10.1016/j.sbi.2012.10.001|pmid=23142576 }}</ref><ref name="scop">{{cite web |title=Double-stranded beta-helix |url=http://scop.berkeley.edu/sunid=51181 |website=SCOPe |access-date=29 November 2021}}</ref> |
||
The first beta-helix was observed in the enzyme [[pectate lyase]], which contains a seven-turn helix that reaches 34 Å (3.4 [[nanometer|nm]]) long. The [[P22 phage]] tail spike protein, a component of the P22 [[bacteriophage]], has 13 turns and in its assembled homo[[protein trimer|trimer]] is 200 Å (20 nm) in length. Its interior is close-packed with no central pore and contains both hydrophobic residues and charged residues neutralized by [[salt bridge]]s. |
The first beta-helix was observed in the enzyme [[pectate lyase]], which contains a seven-turn helix that reaches 34 Å (3.4 [[nanometer|nm]]) long. The [[P22 phage]] tail spike protein, a component of the P22 [[bacteriophage]], has 13 turns and in its assembled homo[[protein trimer|trimer]] is 200 Å (20 nm) in length. Its interior is close-packed with no central pore and contains both hydrophobic residues and charged residues neutralized by [[salt bridge]]s. |
||
Latest revision as of 10:43, 12 November 2023
A beta helix is a tandem protein repeat structure formed by the association of parallel beta sheet in a helical pattern with either two[1] or three[2] faces. The beta helix is a type of solenoid protein domain. The structure is stabilized by inter-strand hydrogen bonds, protein-protein interactions, and sometimes bound metal ions. Both left- and right-handed beta helices have been identified. These structures are distinct from jelly-roll folds, a different protein structure sometimes known as a "double-stranded beta helix".[3][4]
The first beta-helix was observed in the enzyme pectate lyase, which contains a seven-turn helix that reaches 34 Å (3.4 nm) long. The P22 phage tail spike protein, a component of the P22 bacteriophage, has 13 turns and in its assembled homotrimer is 200 Å (20 nm) in length. Its interior is close-packed with no central pore and contains both hydrophobic residues and charged residues neutralized by salt bridges.
Both pectate lyase and P22 tailspike protein contain right-handed helices; left-handed versions have been observed in enzymes such as UDP-N-acetylglucosamine acyltransferase and archaeal carbonic anhydrase.[5] Other proteins that contain beta helices include the antifreeze proteins from the beetle Tenebrio molitor (right-handed)[6] and from the spruce budworm, Choristoneura fumiferana (left-handed),[7] where regularly spaced threonines on the
Beta helices can associate with each other effectively, either face-to-face (mating the faces of their triangular prisms) or end-to-end (forming hydrogen bonds). Hence,
Members of the pentapeptide repeat family have been shown to possess a quadrilateral beta-helix structure.[8]
References
[edit]- ^ "CATH database - folds and homologous superfamilies within the beta 2-solenoid architecture". CATH database.
- ^ "CATH database - folds and homologous superfamilies within the beta 3-solenoid architecture". CATH database. Archived from the original on 26 July 2011.
- ^ Aik, WeiShen; McDonough, Michael A; Thalhammer, Armin; Chowdhury, Rasheduzzaman; Schofield, Christopher J (December 2012). "Role of the jelly-roll fold in substrate binding by 2-oxoglutarate oxygenases". Current Opinion in Structural Biology. 22 (6): 691–700. doi:10.1016/j.sbi.2012.10.001. PMID 23142576.
- ^ "Double-stranded beta-helix". SCOPe. Retrieved 29 November 2021.
- ^ Kisker C, Schindelin H, Alber BE, Ferry JG, Rees DC (May 1996). "A left-hand beta-helix revealed by the crystal structure of a carbonic anhydrase from the archaeon Methanosarcina thermophila". EMBO J. 15 (10): 2323–30. doi:10.1002/j.1460-2075.1996.tb00588.x. PMC 450161. PMID 8665839.
- ^ Liou YC, Tocilj A, Davies PL, Jia Z (July 2000). "Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein". Nature. 406 (6793): 322–4. Bibcode:2000Natur.406..322L. doi:10.1038/35018604. PMID 10917536. S2CID 4385352.
- ^ Leinala EK, Davies PL, Jia Z (May 2002). "Crystal structure of beta-helical antifreeze protein points to a general ice binding model". Structure. 10 (5): 619–27. doi:10.1016/s0969-2126(02)00745-1. PMID 12015145.
- ^ Vetting MW, Hegde SS, Fajardo JE, et al. (January 2006). "Pentapeptide repeat proteins". Biochemistry. 45 (1): 1–10. doi:10.1021/bi052130w. PMC 2566302. PMID 16388575.

