Tetraethyllead: Difference between revisions
+{{Motor fuel}} |
No edit summary |
||
| Line 13: | Line 13: | ||
| OtherNames = Lead tetraethyl<br /> |
| OtherNames = Lead tetraethyl<br /> |
||
Tetraethyl lead<br /> |
Tetraethyl lead<br /> |
||
Tetraetilplomo <br /> |
|||
Tetra-ethyl lead |
Tetra-ethyl lead |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
Revision as of 12:07, 13 August 2012
| |
| |
| Names | |
|---|---|
| IUPAC name
Tetraethylplumbane
| |
| Other names
Lead tetraethyl
Tetraethyl lead | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | TEL |
| 3903146 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.979 |
| EC Number |
|
| 68951 | |
| MeSH | Tetraethyl+lead |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1649 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H20Pb | |
| Molar mass | 323.4 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.653 g cm-3 |
| Melting point | −136 °C (−213 °F; 137 K) |
Refractive index (nD)
|
1.5198 |
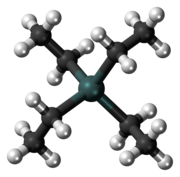
| Structure | |
| Tetrahedral | |
| 0 D | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 73 °C |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetraethyllead (common name tetraethyl lead), abbreviated TEL, is an organolead compound with the formula (CH3CH2)4Pb. Its mixing with gasoline (petrol) as an inexpensive additive beginning in the 1920s allowed octane ratings and thus engine compression to be boosted significantly, increasing power and fuel economy.[1][2] TEL was phased out in view of the toxicity of lead and its deleterious effect on catalytic converters. It is still used as an additive in aviation fuel for piston engine-powered aircraft.
In motor fuel
Tetraethyl lead was extensively used as an additive to gasoline, wherein it served as an effective antiknock agent and prevented exhaust valve and seat wear.
As valve wear preventive
Tetraethyl lead works as a buffer against microwelds forming between the hot exhaust valves and their seats.[3] Once these valves reopen, the microwelds pull apart and leave the valves with a rough surface that would abrade the seats, leading to valve recession. When lead began to be phased out of motor fuel, the automotive industry began specifying hardened valve seats and upgraded exhaust valve materials to prevent valve recession without lead.[4]
As antiknock agent
An engine requires fuel of sufficient octane rating to prevent uncontrolled combustion known as engine knocking ("knock" or "ping").[5] Antiknock agents allow the use of higher compression ratios for greater efficiency[6] and peak power. Adding varying amounts of TEL to gasoline allowed easy, inexpensive control of octane ratings; aviation spirits used in WWII reached 150 octane to enable supercharged engines such as the Rolls-Royce Merlin and Griffon to produce 1500 HP at altitude.[7] In military aviation, TEL manipulation allowed a range of different fuels to be tailored for particular flight conditions, and ease and safety of handling.
The use of TEL in gasoline started in the US, while in Europe, alcohol was initially used. The advantages of leaded gasoline — its higher energy content and storage quality — eventually led to a universal switch to leaded fuel. One of the greatest advantages of TEL over other antiknock agents or the use of high-octane blend stocks is the very low concentrations needed. Typical formulations called for 1 part of prepared TEL to 1260 parts untreated gasoline. Competing antiknock agents must be used in greater amounts, often reducing the energy content of the gasoline.
When used as an antiknock agent, ethanol will cause fuel to absorb moisture from the air. Over time fuel humidity can rise, leading to corrosion in fuel lines. Whereas TEL is highly soluble in gasoline, ethanol is poorly soluble and that solubility decreases as fuel humidity increases. Over time, droplets and pools of water can form in the fuel system creating a risk of fuel line icing. High fuel humidity can also enable biological contamination, as certain bacteria can grow on the surface of the water/gasoline interface, forming bacterial mats in the fuel system. TEL's biocidal properties helped prevent fuel contamination and degradation from bacterial growth.
Phaseout and ban
In most industrialised countries, TEL was phased out of motor fuels in the late 1990s to early 2000s because of concerns over air and soil pollution (e.g., the areas around roads) and the accumulative neurotoxicity of lead. Leaded fuel also spoils catalytic converters, which were introduced in the 1970s to meet tightening emissions regulations. The need for TEL was lessened by several advances in automotive engineering and petroleum chemistry. Other antiknock additives of varying toxicity, such as MMT, MTBE, and ETBE, and safer methods for making higher octane blending stocks such as reformate and iso-octane reduced the need for TEL.
As of June 2011, unleaded automotive gasoline is available almost universally throughout the world and the only countries in which leaded gasoline is the only type available are Burma and Afghanistan; Leaded gasoline also remains available in Algeria, Iraq, North Korea, and Yemen.[8] Lead-replacement additives were scientifically tested and some were approved by the Federation of British Historic Vehicle Clubs at the UK's Motor Industry Research Association in 1999. In Europe, Professor Derek Bryce-Smith was among the first to highlight the potential dangers of TEL and became a leading campaigner for removal of lead additives from petrol.[9] However, leaded motor fuel re-entered the UK market in small quantities from 2000 in response to fervent lobbying from classic-car organisations who contended their vehicles would be rendered useless without leaded fuel. The lead content is up to 0.15 grams per litre, and Bayford & Co are the only wholesale supplier.[10]
Vehicles designed and built to run on leaded fuel may require modification to run on unleaded gasoline. These modifications fall into two categories: those required for physical compatibility with unleaded fuel, and those performed to compensate for the relatively low octane of early unleaded fuels. Physical compatibility requires the installation of hardened exhaust valves and seats. Compatibility with reduced octane was addressed by reducing compression, generally by installing thicker cylinder head gaskets and/or rebuilding the engine with compression-reducing pistons. The availability of high-octane unleaded gasolines has reduced or eliminated the need to alter engines' compression ratios.
Leaded-fuel bans for road vehicles came into effect as follows:
- Europe
- Austria: 1989
- Serbia: 2010
- Bosnia and Herzegovina: 2009
- EU: 1. January 2000
- Iceland:
- Monaco: 2000
- Norway:
- Switzerland: 2000
- Poland: 2005
- Africa
Leaded petrol was supposed to be completely phased out continent-wide on 1 January 2006, following a ban initiated from the 2002 Earth Summit.[11] However, leaded fuel remains available in Algeria.[8]
- North America
- Canada: 1993
- USA: 1995
- California: 1992
- Bahamas:
- Belize:
- Bermuda:
- Costa Rica:
- Dominican Republic:
- El Salvador:
- Guatemala:
- Haiti:
- Honduras:
- Mexico: 1998
- Nicaragua:
- Puerto Rico:
- Trinidad and Tobago: 2000
- South America
- Argentina:
- Bolivia:
- Brazil: 1989
- Chile:
- Colombia:
- Peru: 2004
- Asia
- Japan: 1986
- Hong Kong: 1999
- Malaysia: 2000
- Singapore: 1998
- South Korea: 1993
- Sri Lanka: 1999
- Thailand:
- Bangladesh:
- Taiwan: 2000
- China: 2000
- Philippines: 2000
- India: 2000
- Nepal: 2000
- Indonesia: 2006
- Oceania
- Australia: 2002[12]
- New Zealand: 1996
- Guam:
In race vehicles
Until recently, leaded fuel was used in professional auto racing. NASCAR switched to unleaded fuel in 2008 after years of research, spurred when blood tests of NASCAR teams revealed elevated blood lead levels.[13][14] An initial test in 2005 with usually dependable motors had led to five cars retiring with engine troubles in a single race.[15]
In aviation fuel
TEL remains an ingredient of 100 octane avgas for piston-engine aircraft. The United States Environmental Protection Agency and others are working on an economically feasible replacement for leaded avgas. The current formulation of 100LL (low lead) aviation gasoline contains 2.12 grams of TEL per gallon, half the amount of the previous 100 octane avgas (at 4.24 grams per gallon),[16] but far more than the 0.1 gram per gallon permitted in automotive leaded gasoline or the 0.001 grams per gallon in automotive unleaded gasoline sold in the United States.[17]
Alternative antiknock agents
Antiknock agents are classed as "high-percentage" additives, such as alcohol, and "low-percentage" additives based on heavy elements. Since the main problem with TEL is its lead content, many alternative additives that contain less poisonous metals have been examined. A manganese-carrying additive, methylcyclopentadienyl manganese tricarbonyl (MMT or methylcymantrene), was used for a time as an antiknock agent, though its safety is controversial and it has been the subject of bans and lawsuits. Ferrocene, an organometallic compound of iron, has also been reported[by whom?] as an effective antiknock agent.
High-percentage additives are organic compounds that do not contain metals, but require much higher blending ratios, such as 20–30% for benzene and ethanol. It had also been established by 1921 that ethanol was an effective antiknock agent, but TEL was introduced instead mainly for commercial reasons.[18] Oxygenates such as TAME derived from natural gas, MTBE made from methanol, and ethanol-derived ETBE, have largely supplanted TEL. MTBE has environmental risks of its own and there are also bans on its use. ETBE requires more expensive ethanol as a starting material.
Improvements of the gasoline itself decrease the need for added antiknock agents. Synthetic iso-octane and alkylate are examples of such blending stocks. Benzene and other high-octane aromatics can be also blended to raise the octane number, but they are disfavored today because of toxicity and carcinogenicity.
Synthesis and properties
TEL is produced by reacting chloroethane with a sodium–lead alloy.[5]
- 4 NaPb + 4 CH3CH2Cl → (CH3CH2)4Pb + 4 NaCl + 3 Pb
Despite decades of research, no reactions were found to improve upon this rather difficult process that involves metallic sodium. The product, TEL, is a viscous colorless liquid. Because TEL is charge neutral and contains an exterior of alkyl groups, it is highly lipophilic and soluble in petrol (gasoline).
A process with lithium was developed, but never put into practice. A related compound, tetramethyllead, was commercially produced by a different electrolytic reaction.[5]
Reactions
A noteworthy feature of TEL is the weakness of its four C–Pb bonds. At the temperatures found in internal combustion engines,(CH3CH2)4Pb decomposes completely into lead and lead oxides as well as combustible, short-lived ethyl radicals. Lead and lead oxide scavenge radical intermediates in combustion reactions. This prevents ignition of unburnt fuel during the engine's exhaust stroke.[5] Lead itself is the reactive antiknock agent, and TEL serves as a gasoline-soluble lead carrier.[5] When (CH3CH2)4Pb burns, it produces not only carbon dioxide and water, but also lead:
- (CH3CH2)4Pb + 13 O2 → 8 CO2 + 10 H2O + Pb
This lead can oxidize further to give species such as lead(II) oxide:
- 2 Pb + O2 → 2 PbO
The Pb and PbO would quickly over-accumulate and destroy an engine. For this reason, the lead scavengers 1,2-dibromoethane and 1,2-dichloroethane are used in conjunction with TEL—these agents form volatile lead(II) bromide and lead(II) chloride, respectively, which are flushed from the engine and into the air.
Formulation of ethyl fluid

TEL was supplied for mixing with raw gasoline in the form of ethyl fluid, which was TEL blended together with the lead scavengers 1,2-dibromoethane and 1,2-dichloroethane. Ethyl fluid also contained a reddish dye to distinguish treated from untreated gasoline and discourage the use of leaded gasoline for other purposes such as cleaning.
Ethyl fluid was added to gasoline at rate of 1:1260, usually at the refinery.[citation needed] Because of the widespread use and toxic nature of ethyl fluid, the Ethyl Corporation developed an expertise in its safe handling. In the 1920s, before safety procedures were yet developed, some 17 workers for the Ethyl Corporation and Standard Oil died [citation needed] from the effects of exposure to lead.
The formula for ethyl fluid is:[5]
- Tetraethyllead 61.45%
- 1,2-Dibromoethane 17.85%
- 1,2-Dichloroethane 18.80%
- Inerts & dye 1.90%
Dibromoethane and dichloroethane act in a synergistic manner, where a particular mixing ratio provides the best lead scavenging ability.[5]
Toxicity
Lead pollution from engine exhaust is dispersed into the air and into the vicinity of roads and easily inhaled. Contact with concentrated TEL leads to acute lead poisoning:
Lead is a toxic metal that accumulates and has subtle and insidious neurotoxic effects especially at low exposure levels, such as low IQ and antisocial behavior. It has particularly harmful effects on children. These concerns eventually led to the ban on TEL in automobile gasoline in many countries. Some neurologists have speculated that the lead phaseout may have caused average IQ levels to rise by several points in the US (by reducing cumulative brain damage throughout the population, especially in the young). For the entire US population, during and after the TEL phaseout, the mean blood lead level dropped from 16
A statistically significant correlation has been found between the usage rate of leaded gasoline and violent crime: taking into account a 22-year time lag, the violent crime curve virtually tracks the lead exposure curve.[21][19] After the ban on TEL, blood lead levels in US children dramatically decreased.[19]
Although leaded gasoline is largely gone in North America, it has left high concentrations of lead in the soil adjacent to roads that were constructed prior to its phaseout. Children are particularly at risk if they consume this.[citation needed]
History
Tetraethyllead was first discovered by a German chemist in 1854, but remained commercially unused for many years.[18] In 1921, TEL was found to be an effective antiknock agent by Thomas Midgley, working under Charles Kettering at General Motors Corporation Research.[22] General Motors patented the use of TEL as a knocking agent and called it "Ethyl" in its marketing materials, thereby avoiding the negative connotation of the word "lead".[18] By 1923, leaded gasoline was being sold.[23] In 1924, Standard Oil of New Jersey (ESSO/EXXON) and General Motors created the Ethyl Gasoline Corporation to produce and market TEL.
The toxicity of concentrated TEL was recognized early on, as lead had been recognized since the 19th century as a dangerous substance which could cause lead poisoning.[23] In 1924, a public controversy arose over the "loony gas," after several workers died and others went insane in a refinery in New Jersey and a DuPont facility in Ohio.[23] However, for two years prior to this controversy, several public health experts including Alice Hamilton engaged Midgley and Kettering with letters warning of the dangers to public health of the proposed plan.[23] After the death of the workers, dozens of newspapers reported on the issue.[23][24] In 1925, the sales of TEL were suspended for one year to conduct a hazard assessment.[5][18]
The U.S. Public Health Service conducted a conference in 1925. The conference was initially expected to last for several days, but reportedly the conference decided that evaluating presentations on alternative anti-knock agents was not "its province", so it lasted a single day.[23] Kettering and Midgley stated that no alternatives for anti-knocking were available, although private memos showed discussion of such agents.[23] One commonly discussed agent was ethanol, although it was not as cheap.[23] The Public Health Service created a committee which reviewed a government-sponsored study of workers and an Ethyl lab test, and concluded that while leaded gasoline should not be banned, it should continue to be investigated.[23] The low concentrations present in gasoline and exhaust were not perceived as immediately dangerous. A U.S. Surgeon General committee issued a report in 1926 that concluded there was no real evidence that the sale of TEL was hazardous to human health but urged further study.[18] In the years that followed, research was heavily funded by the lead industry; in 1943, Randolph Byers found children with lead poisoning had behavior problems, but he was threatened with a lawsuit and the research ended.[23]
In the late 1920s, Dr. Robert Kehoe of the University of Cincinnati was the Ethyl Corporation's chief medical consultant. In 1928, Dr. Kehoe expressed the opinion that there was no basis for concluding that leaded fuels posed any health threat.[18] He convinced the Surgeon General that the dose–response relationship of lead was "no effect" below a certain threshold.[25] As the head of Kettering Laboratories for many years, Kehoe would become a chief promoter of the safety of TEL, an influence that did not begin to wane until about the early 1960s. But by the 1970s, the general opinion of the safety of TEL would change, and by 1976 the U.S. government would begin to require the phaseout of this product.
As early as the late 1940s and early 1950s, Clair Patterson accidentally discovered the pollution caused by TEL in the environment while determining the age of the earth. As he attempted to measure lead content of very old rocks, and the time it took uranium to decay into lead, the readings were made inaccurate by lead in the environment that contaminated his samples. He was then forced to work in a clean room to keep his samples uncontaminated by environmental pollution of lead. After coming up with a fairly accurate estimate of the age of the earth, he turned to investigating the lead contamination problem by examining ice cores from countries such as Greenland. He realized that the lead contamination in the environment dated from about the time that TEL became widely used as a fuel additive in gasoline. Being aware of the health dangers posed by lead and suspicious of the pollution caused by TEL, he became one of the earliest and most effective opponents of its use.[26]
In the 1960s the first clinical works were published proving the toxicity of this compound in humans, e.g. by Mirosław Jan Stasik.[27]
In the 1970s Herbert Needleman found that higher blood levels in children were correlated with decreased school performance. Needleman was repeatedly accused of scientific misconduct by individuals within the lead industry, but he was eventually cleared by a scientific advisory council.[23]
In the U.S. in 1972, the EPA launched an initiative to phase out leaded gasoline based on a regulation under the authority of the Clean Air Act Extension of 1970. Ethyl Corp's response was to sue the EPA. Although the EPA's regulation was initially dismissed,[23] the EPA won the case on appeal, so the TEL phaseout began in 1976 and was completed by 1986. A 1994 study indicated that the concentration of lead in the blood of the U.S. population had dropped 78% from 1976 to 1991.[28]
By 2000, the TEL industry had moved the major portion of their sales to developing countries whose governments they lobbied against phasing out leaded gasoline.[18] Leaded gasoline was withdrawn entirely from the European Union market on 1 January 2000, although it had been banned much earlier in most member states. Other countries also phased out TEL.[29]
In literature
The effects of tetraethyllead are described in The Roman Hat Mystery by Ellery Queen.
See also
References
- ^ "TETRA-ETHYL LEAD AS AN ADDITION TO PETROL". British Medical Journal. 1 (3504): 366. 3 March 1928. doi:10.1136/bmj.1.3504.366.
- ^ Popular Science (October 1987 ed.), p. 94 http://books.google.co.nz/books?id=oAAAAAAAMBAJ&&pg=PA94
{{citation}}: Missing or empty|title=(help) - ^ Engine Performance Facts and Fixes: No-Lead Fuel
- ^ 1973 Cleaner Air System Highlights: Hardened exhaust valve seats
- ^ a b c d e f g h Seyferth, D. (2003). "The Rise and Fall of Tetraethyllead. 2". Organometallics. 22 (25): 5154–5178. doi:10.1021/om030621b.
- ^ Caris, D. F. and Nelson, E. E. (1959). A New Look at High Compression Engines SAE Trans.
- ^ I Kept No Diary. Air Commodore F.R. Banks, 1978, ISBN 0-904543-9-7
- ^ a b Robert Taylor (17 June 2011). "Countries where Leaded Petrol is Possibly Still Sold for Road Use". The LEAD Group.
- ^ Obituary: Derek Bryce Smith
- ^ "Fuel Information". Federation of British Historic Vehicle Clubs.
- ^ Geoffrey Lean (1 January 2006). "UN hails green triumph as leaded petrol is banned throughout Africa". The Independent.
- ^ Australia Cuts Sulfur Content in Transport Fuels
- ^ O'Neil, J; Steele, G; McNair, CS; Matusiak, MM; Madlem, J (2006). "Blood lead levels in NASCAR Nextel Cup Teams". Journal of occupational and environmental hygiene. 3 (2): 67–71. doi:10.1080/15459620500471221. PMID 16361219.
- ^ "NASCAR to Use Unleaded Fuel in 2008".
- ^ http://www.startribune.com/sports/motorsports/12092486.html
- ^ "Issues Related to Lead in Avgas". Aircraft Owners and Pilots Association.
- ^ "Modifications / Octane / Lead Content / Fuel Specs / Limitations / Certification". Petersen Aviation Inc.
- ^ a b c d e f g Kitman, J. (Mar. 2, 2000). "The Secret History of Lead. The Nation. Retrieved 8-17-2009.
- ^ a b c Reyes, J. W. (2007). "The Impact of Childhood Lead Exposure on Crime". National Bureau of Economic Research. "a" ref citing Pirkle, Brody, et. al (1994). Retrieved 8-17-2009.
- ^ Lanphear, Bruce P.; Hornung, Richard; Khoury, Jane; Yolton, Kimberly; Baghurst, Peter; Bellinger, David C.; Canfield, Richard L.; Dietrich, Kim N.; Bornschein, Robert (2005). "Low-Level Environmental Lead Exposure and Children's Intellectual Function: An International Pooled Analysis". Environmental Health Perspectives. 113 (7): 894–9. doi:10.1289/ehp.7688. PMC 1257652. PMID 16002379.
- ^ Ban on leaded petrol 'has cut crime rates around the world'
- ^ "Leaded Gasoline, Safe Refrigeration, and Thomas Midgley, Jr." Chapter 6 in S. Bertsch McGrayne. Prometheans in the Lab. McGraw-Hill: New York, 2002. ISBN 0-07-140795-2
- ^ a b c d e f g h i j k l Kovarik W (2005). "Ethyl-leaded gasoline: how a classic occupational disease became an international public health disaster". Int J Occup Environ Health. 11 (4): 384–97. PMID 16350473.Free full-text (registration required)
- ^ TEL-related deaths
- ^ Bryson, Christopher (2004). The Fluoride Deception, p. 41. Seven Stories Press. Citing historian Lynne Snyder.
- ^ Bryson, Bill (2003). "Getting the Lead Out", Chapter 10 in A Short History of Nearly Everything. Broadway Books: New York. ISBN 0-7679-0818-X
- ^ Stasik et al.: Acute Tetraethyllead Poisoning. Arch. Toxikol. 24. 283-291,1969
- ^ Pirkle, J. L.; Brody, D. J.; Gunter, E. W.; et al. (1994). "The Decline in Blood Lead Levels in the United States: The National Health and Nutrition Examination Surveys (NHANES)". J. Am. Med. Assoc. 272 (4): 284–291. doi:10.1001/jama.272.4.284.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "The Case for Banning Lead in Gasoline" (PDF). Manufacturers of Emission Controls Association (MECA). January 2003. Retrieved 7 June 2012.
External links
- U.S. Gov't, Agency for Toxic Substances and Disease Registry. Case Studies in Environmental Medicine (CSEM): Lead Toxicity
- U.S. Gov't, Agency for Toxic Substances and Disease Registry. ToxFAQs: Lead
- Australian Government, National Pollutant Inventory - Lead and Lead Compounds Fact Sheet
- Kovarik, Bill (1999). Charles F. Kettering and the 1921 Discovery of Tetraethyl Lead
- True unleaded alternative for 100LL needed for general aviation

