O-Xylene: Difference between revisions
→Production and use: bromination |
→Production and use: ref |
||
| Line 87: | Line 87: | ||
[[Petroleum]] contains about one weight percent xylenes. Most ''o''-xylene is produced by cracking [[petroleum]], which affords a distribution of aromatic compounds, including xylene isomers. ''m''-Xylene is isomerized to ''o''-xylene. Net production was approximately 500,000 tons in the year 2000. |
[[Petroleum]] contains about one weight percent xylenes. Most ''o''-xylene is produced by cracking [[petroleum]], which affords a distribution of aromatic compounds, including xylene isomers. ''m''-Xylene is isomerized to ''o''-xylene. Net production was approximately 500,000 tons in the year 2000. |
||
''o''-Xylene is largely used in the production of [[phthalic anhydride]], which is a precursor to many materials, drugs, and other chemicals.<ref name="Ullmann"/> Related to their easy oxidation, the methyl groups are susceptible to halogenation. When treated with bromine, these groups are brominated, leading ultimately to [[Tetrabromo-o-xylene|the tetrabromide]]: |
''o''-Xylene is largely used in the production of [[phthalic anhydride]], which is a precursor to many materials, drugs, and other chemicals.<ref name="Ullmann"/> Related to their easy oxidation, the methyl groups are susceptible to halogenation. When treated with bromine, these groups are brominated, leading ultimately to [[Tetrabromo-o-xylene|the tetrabromide]]:<ref name=OS>{{cite journal |doi=10.15227/orgsyn.034.0082|title=o-Phthalaldehyde|journal=Organic Syntheses|year=1954|first1=J. C.|last1=Bill|first2=D. S.|last2=Tarbell |volume=34|page=82}}</ref> |
||
:C<sub>6</sub>H<sub>4</sub>(CH<sub>3</sub>)<sub>2</sub> + 4 Br<sub>2</sub> → C<sub>6</sub>H<sub>4</sub>(CHBr<sub>2</sub>)<sub>2</sub> + 4 HBr |
|||
==Toxicity and exposure== |
==Toxicity and exposure== |
||
Revision as of 05:01, 7 November 2020
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Xylene | |||
| Systematic IUPAC name
2-Methyltoluene | |||
| Other names
ortho-Xylene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.203 | ||
| KEGG | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H10 | |||
| Molar mass | 106.168 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.88 g/ml | ||
| Melting point | −24 °C (−11 °F; 249 K) | ||
| Boiling point | 144.4 °C (291.9 °F; 417.5 K) | ||
| 0.02% (20°C)[1] | |||
| Solubility in ethanol | very soluble | ||
| Solubility in diethyl ether | very soluble | ||
| Vapor pressure | 7 mmHg (20°C)[1] | ||
| -77.78·10−6 cm3/mol | |||
Refractive index (nD)
|
1.50545 | ||
| Viscosity | 1.1049 cP at 0 °C 0.8102 cP at 20 °C | ||
| Structure | |||
| 0.64 D [2] | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 32 °C (90 °F; 305 K) | ||
| 463 °C (865 °F; 736 K)[3] | |||
| Explosive limits | 0.9%-6.7%[1] | ||
Threshold limit value (TLV)
|
100 ppm[3] (TWA), 150 ppm[3] (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published)
|
6125 ppm (rat, 12 hr) 6125 ppm (human, 12 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 100 ppm (435 mg/m3)[1] | ||
REL (Recommended)
|
TWA 100 ppm (435 mg/m3) ST 150 ppm (655 mg/m3)[1] | ||
IDLH (Immediate danger)
|
900 ppm[1] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related aromatic hydrocarbons
|
m-xylene p-xylene toluene | ||
| Supplementary data page | |||
| O-Xylene (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
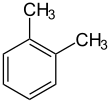
o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2. with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration). It is a constitutional isomer of m-xylene and p-xylene, the mixture being called xylene or xylenes. o-Xylene is a colorless slightly oily flammable liquid.[5]
Production and use
Petroleum contains about one weight percent xylenes. Most o-xylene is produced by cracking petroleum, which affords a distribution of aromatic compounds, including xylene isomers. m-Xylene is isomerized to o-xylene. Net production was approximately 500,000 tons in the year 2000.
o-Xylene is largely used in the production of phthalic anhydride, which is a precursor to many materials, drugs, and other chemicals.[5] Related to their easy oxidation, the methyl groups are susceptible to halogenation. When treated with bromine, these groups are brominated, leading ultimately to the tetrabromide:[6]
- C6H4(CH3)2 + 4 Br2 → C6H4(CHBr2)2 + 4 HBr
Toxicity and exposure
Xylenes are not acutely toxic, for example the LD50 (rat, oral) is 4300 mg/kg. Effects vary with animal and xylene isomer. Concerns with xylenes focus on narcotic effects.[5]
References
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0668". National Institute for Occupational Safety and Health (NIOSH).
- ^ Rudolph, H.D.; Walzer, K.; Krutzik, Irmhild (1973). "Microwave spectrum, barrier for methyl rotation, methyl conformation, and dipole moment of ortho-xylene". Journal of Molecular Spectroscopy. 47 (2): 314. Bibcode:1973JMoSp..47..314R. doi:10.1016/0022-2852(73)90016-7.
- ^ a b c "o-Xylene". International Chemical Safety Cards. ICSC/NIOSH. July 1, 2014.
- ^ "Xylene (o-, m-, p-isomers)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Fabri, Jörg; Graeser, Ulrich; Simo, Thomas A. (2000). "Xylenes". Xylenes]. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_433. ISBN 3527306730.
- ^ Bill, J. C.; Tarbell, D. S. (1954). "o-Phthalaldehyde". Organic Syntheses. 34: 82. doi:10.15227/orgsyn.034.0082.