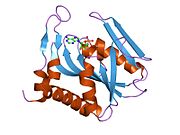
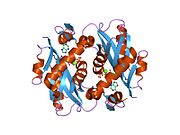
Adenine phosphoribosyltransferase
Template:PBB Adenine phosphoribosyltransferase (APRTase) is an enzyme encoded by the APRT gene, found in humans on chromosome 16.[1] It is part of the Type I PRTase family and is involved in the nucleotide salvage pathway, which provides an alternative to nucleotide biosynthesis de novo in humans and most other animals.[2] In parasitic protozoa such as giardia, APRTase provides the sole mechanism by which adenine can be produced. [3] APRTase deficiency contributes to the formation of kidney stones (urolithiasis) and to potential kidney failure.[4]
Biological Function
APRTase catalyzes the following reaction in the purine nucleotide salvage pathway:
Adenine + Phosphoribosyl Pyrophosphate (PRPP) → Adenylate (AMP) + Pyrophosphate (PPi)

In organisms that can synthesize purines de novo, the nucleotide salvage pathway provides an alternative that is energetically more efficient. It can salvage adenine from the polyamine biosynthetic pathway or from dietary sources of purines.[2] Although APRTase is functionally redundant in these organisms, it becomes more important during periods of rapid growth, such as embryogenesis and tumor growth.[5] It is constitutively expressed in all mammalian tissue.[6]
In protozoan parasites, the nucleotide salvage pathway provides the sole means for nucleotide synthesis. Since the consequences of APRTase deficiency in humans is comparatively mild and treatable, it may be possible to treat certain parasitic infections by targeting APRTase function.[7]
In plants, as in other organisms, ARPTase functions primarily for the synthesis of adenylate. It has the unique ability to metabolize cytokinins -- a plant hormone that can exist as a base, nucleotide, or nucleoside -- into adenylate nucleotides.[8]
APRT is functionally related to hypoxanthine-guanine phosphoribosyltransferase (HPRT).
Structure
APRTase is a homodimer, with 179 amino acid residues per monomer. Each monomer contains the following regions:
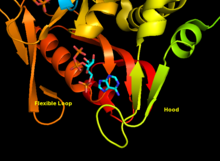
- "Core" domain (residues 33-169) with five parallel
β -sheets - "Hood" domain (residues 5-34) with 2
α -helices and 2β -sheets - "Flexible loop" domain (residues 95-113) with 2 antiparallel
β -sheets [6]
The core is highly conserved across many PRTases. The hood, which contains the adenine binding site, has more variability within the family of enzymes. A 13-residue motif comprises the PRPP binding region and involves two adjacent acidic residues and at least one surrounding hydrophobic residue. [9]
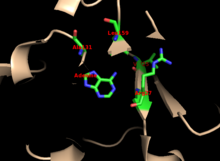
The enzyme's specificity for adenine involves hydrophobic residues Ala131 and Leu159 in the core domain. In humans specifically, two residues in the hood domain hydrogen bond with the purine for further specificity: Val25 with the hydrogens on N6, and Arg27 with N1. Although the flexible loop does not interact with the hood during purine recognition, it is thought to close over the active site and sequester the reaction from solvents. [6]
Most research on APRTase reports that Mg2+ is essential for phosphoribosyl transfer, and this is conserved across Type I PRTases. [8] However, a recent effort to resolve the structure of human APRTase was unable to locate a single site for Mg2+, but did find evidence to suggest a Cl- atom near [[Tryptophan|Trp98}. Despite the difficulty of placing Mg2+, it is generally accepted that the catalytic mechanism is dependent on this ion. [2]
Mechanism
APRTase proceeds via a bi bi ordered sequential mechanism, involving the formation of a ternary complex. The enzyme first binds PRPP, followed by adenine. After the phosphoribosyl transfer occurs, pyrophosphate leaves first, followed by AMP. Kinetic studies indicate that the phosphoribosyl transfer is relatively fast, while the product release (particularly the release of AMP) is rate-limiting.[10]
In human APRTase, it is thought that adenine's N9 proton is abstracted by Glu104 to form an oxacarbenium transition state. This functions as the nucleophile to attack the anomeric carbon of PRPP, forming AMP and displacing pyrophosphate from PRPP. The mechanism of APRTase is generally consistent with that of other PRTases, which conserve the function of displacing PRPP's
Deficiency
2,8-Dihydroxy-adenine urolithiasis is also known as "adenine phosphoribosyltransferase deficiency". When APRTase has reduced or nonexistent activity, adenine accumulates from other pathways. It is degraded by xanthine dehydrogenase to 2,8-dihydroxyadenine (DHA). Although DHA is protein-bound in plasma, it has poor solubility in urine and gradually precipitates in kidney tubules, leading to the formation of kidney stones (urolithiasis). If left untreated, the condition can eventually produce kidney failure. Cite error: The opening <ref> tag is malformed or has a bad name (see the help page).
ARPTase deficiency was first diagnosed in the UK in 1976. Since then, two categories of APRTase deficiency have been defined in humans.[11]
Type I deficiency results in a complete loss of APRTase activity and can occur in patients that are homozygous or compound heterozygous for various mutations.[12] Sequencing has revealed many different mutations that can account for Type 1, including missense mutations, nonsense mutations, a duplicated set of 4 base pairs in exon 3, [13] and a single thymine insertion in intron 4.[14] These mutations cause effects that are clustered into three main areas: in the binding of PRPP's
Type II deficiency causes APRTase to have a reduced affinity for PRPP, resulting in a tenfold increase in the KM value. [2] It has been observed and studied primarily in Japan. [14]
A diagnosis of APRTase deficiency can be made by analyzing kidney stones, measuring DHA concentrations in urine, or analyzing APRTase activity in erythrocytes. It is treatable with regular doses of allopurinol or febuxostat, which inhibit xanthine dehydrogenase activity to prevent the accumulation and precipitation of DHA. [15] The condition can also be attenuated with a low-purine diet and high fluid intake. [11]
References
- ^ Valaperta, R; Rizzo, V; Lombardi, F; Verdelli, C; Piccoli, M; Ghiroldi, A; Creo, P; Colombo, A; Valisi, M; Margiotta, E; Panella, R; Costa, E (1 July 2014). "Adenine phosphoribosyltransferase (APRT) deficiency: identification of a novel nonsense mutation". BMC nephrology. 15: 102. PMID 24986359.
- ^ a b c d e Silva, CH; Silva, M; Iulek, J; Thiemann, OH (June 2008). "Structural complexes of human adenine phosphoribosyltransferase reveal novel features of the APRT catalytic mechanism". Journal of biomolecular structure & dynamics. 25 (6): 589–97. PMID 18399692.
- ^ Sarver, AE; Wang, CC (18 October 2002). "The adenine phosphoribosyltransferase from Giardia lamblia has a unique reaction mechanism and unusual substrate binding properties". The Journal of biological chemistry. 277 (42): 39973–80. PMID 12171924.
- ^ Shi, Wuxian; Tanaka, Kelly; Crother, Timothy; Taylor, Milton; Almo, Steven; Schramm, Vern (2001). "Structural Analysis of Adenine Phosphoribosyltransferase from Saccharomyces cerevisiae". Biochemistry. 40 (36): 10800–10809.
- ^ Bashor, C; Denu, JM; Brennan, RG; Ullman, B (26 March 2002). "Kinetic mechanism of adenine phosphoribosyltransferase from Leishmania donovani". Biochemistry. 41 (12): 4020–31. PMID 11900545.
- ^ a b c d Silva, M; Silva, CH; Iulek, J; Thiemann, OH (22 June 2004). "Three-dimensional structure of human adenine phosphoribosyltransferase and its relation to DHA-urolithiasis". Biochemistry. 43 (24): 7663–71. PMID 15196008.
- ^ Shi, W; Sarver, AE; Wang, CC; Tanaka, KS; Almo, SC; Schramm, VL (18 October 2002). "Closed site complexes of adenine phosphoribosyltransferase from Giardia lamblia reveal a mechanism of ribosyl migration". The Journal of biological chemistry. 277 (42): 39981–8. PMID 12171925.
- ^ a b Allen, M; Qin, W; Moreau, F; Moffatt, B (May 2002). "Adenine phosphoribosyltransferase isoforms of Arabidopsis and their potential contributions to adenine and cytokinin metabolism". Physiologia plantarum. 115 (1): 56–68. PMID 12010467.
- ^ Kukimoto-Niino, Mutsuko; et al. (March 2005). "Crystal structure of a predicted phosphoribosyltransferase (TT1426) from Thermus thermophilus HB8 at 2.01 Å resolution". Protein Science. 14 (3): 823–7. PMID 2279281.
{{cite journal}}: Explicit use of et al. in:|last2=(help) - ^ Bashor, C; Denu, JM; Brennan, RG; Ullman, B (26 March 2002). "Kinetic mechanism of adenine phosphoribosyltransferase from Leishmania donovani". Biochemistry. 41 (12): 4020–31. PMID 11900545.
- ^ a b Cassidy, MJ; McCulloch, T; Fairbanks, LD; Simmonds, HA (March 2004). "Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient". Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 19 (3): 736–8. PMID 14767036.
- ^ Bollée, G; Harambat, J; Bensman, A; Knebelmann, B; Daudon, M; Ceballos-Picot, I (September 2012). "Adenine phosphoribosyltransferase deficiency". Clinical journal of the American Society of Nephrology : CJASN. 7 (9): 1521–7. PMID 22700886.
- ^ Kamatani, N; Hakoda, M; Otsuka, S; Yoshikawa, H; Kashiwazaki, S (July 1992). "Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients". The Journal of clinical investigation. 90 (1): 130–5. PMID 1353080.
- ^ a b c Bollée, G; Dollinger, C; Boutaud, L; Guillemot, D; Bensman, A; Harambat, J; Deteix, P; Daudon, M; Knebelmann, B; Ceballos-Picot, I (April 2010). "Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency". Journal of the American Society of Nephrology : JASN. 21 (4): 679–88. PMID 20150536.
- ^ Edvardsson, VO; Palsson, R; Sahota, A; Pagon, RA; Adam, MP; Ardinger, HH; Wallace, SE; Amemiya, A; Bean, LJH; Bird, TD; Fong, CT; Mefford, HC; Smith, RJH; Stephens, K (1993). "Adenine Phosphoribosyltransferase Deficiency". PMID 22934314.
{{cite journal}}: Cite journal requires|journal=(help)
Further reading
- Tischfield JA, Engle SJ, Gupta PK; et al. (1995). "Germline and somatic mutation at the APRT locus of mice and man". Adv. Exp. Med. Biol. 370: 661–4. doi:10.1007/978-1-4615-2584-4_137. PMID 7660991.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Takeuchi H, Kaneko Y, Fujita J, Yoshida O (1993). "A case of a compound heterozygote for adenine phosphoribosyltransferase deficiency (APRT*J/APRT*Q0) leading to 2,8-dihydroxyadenine urolithiasis: review of the reported cases with 2,8-dihydroxyadenine stones in Japan". J. Urol. 149 (4): 824–6. PMID 8455250.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ludwig H, Kuzmits R, Pietschmann H, Müller MM (1980). "Enzymes of the purine interconversion system in chronic lymphatic leukemia: decreased purine nucleoside phosphorylase and adenosine deaminase activity". Blut. 39 (5): 309–15. doi:10.1007/BF01014193. PMID 116697.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Johnson LA, Gordon RB, Emmerson BT (1977). "Adenine phosphoribosyltransferase: a simple spectrophotometric assay and the incidence of mutation in the normal population". Biochem. Genet. 15 (3–4): 265–72. doi:10.1007/BF00484458. PMID 869896.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kamatani N, Hakoda M, Otsuka S; et al. (1992). "Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients". J. Clin. Invest. 90 (1): 130–5. doi:10.1172/JCI115825. PMC 443071. PMID 1353080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Chen J, Sahota A, Laxdal T; et al. (1992). "Identification of a single missense mutation in the adenine phosphoribosyltransferase (APRT) gene from five Icelandic patients and a British patient". Am. J. Hum. Genet. 49 (6): 1306–11. PMC 1686459. PMID 1746557.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Mimori A, Hidaka Y, Wu VC; et al. (1991). "A mutant allele common to the type I adenine phosphoribosyltransferase deficiency in Japanese subjects". Am. J. Hum. Genet. 48 (1): 103–7. PMC 1682758. PMID 1985452.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Chen J, Sahota A, Stambrook PJ, Tischfield JA (1991). "Polymerase chain reaction amplification and sequence analysis of human mutant adenine phosphoribosyltransferase genes: the nature and frequency of errors caused by Taq DNA polymerase". Mutat. Res. 249 (1): 169–76. doi:10.1016/0027-5107(91)90143-C. PMID 2067530.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gathof BS, Sahota A, Gresser U; et al. (1992). "Identification of a splice mutation at the adenine phosphoribosyltransferase locus in a German family". Klin. Wochenschr. 69 (24): 1152–5. doi:10.1007/BF01815434. PMID 2135300.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kamatani N, Kuroshima S, Hakoda M; et al. (1990). "Crossovers within a short DNA sequence indicate a long evolutionary history of the APRT*J mutation". Hum. Genet. 85 (6): 600–4. doi:10.1007/BF00193582. PMID 2227951.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kamatani N, Kuroshima S, Terai C; et al. (1989). "Detection of an amino acid substitution in the mutant enzyme for a special type of adenine phosphoribosyltransferase (APRT) deficiency by sequence-specific protein cleavage". Am. J. Hum. Genet. 45 (2): 325–31. PMC 1683345. PMID 2502918.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hidaka Y, Tarlé SA, Fujimori S; et al. (1988). "Human adenine phosphoribosyltransferase deficiency. Demonstration of a single mutant allele common to the Japanese". J. Clin. Invest. 81 (3): 945–50. doi:10.1172/JCI113408. PMC 442550. PMID 3343350.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wilson JM, O'Toole TE, Argos P; et al. (1986). "Human adenine phosphoribosyltransferase. Complete amino acid sequence of the erythrocyte enzyme". J. Biol. Chem. 261 (29): 13677–83. PMID 3531209.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Broderick TP, Schaff DA, Bertino AM; et al. (1987). "Comparative anatomy of the human APRT gene and enzyme: nucleotide sequence divergence and conservation of a nonrandom CpG dinucleotide arrangement". Proc. Natl. Acad. Sci. U.S.A. 84 (10): 3349–53. doi:10.1073/pnas.84.10.3349. PMC 304867. PMID 3554238.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hidaka Y, Palella TD, O'Toole TE; et al. (1987). "Human adenine phosphoribosyltransferase. Identification of allelic mutations at the nucleotide level as a cause of complete deficiency of the enzyme". J. Clin. Invest. 80 (5): 1409–15. doi:10.1172/JCI113219. PMC 442397. PMID 3680503.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hidaka Y, Tarlé SA, O'Toole TE; et al. (1988). "Nucleotide sequence of the human APRT gene". Nucleic Acids Res. 15 (21): 9086. doi:10.1093/nar/15.21.9086. PMC 306432. PMID 3684585.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Chen J, Sahota A, Martin GF; et al. (1993). "Analysis of germline and in vivo somatic mutations in the human adenine phosphoribosyltransferase gene: mutational hot spots at the intron 4 splice donor site and at codon 87". Mutat. Res. 287 (2): 217–25. doi:10.1016/0027-5107(93)90014-7. PMID 7685481.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sahota A, Chen J, Boyadjiev SA; et al. (1994). "Missense mutation in the adenine phosphoribosyltransferase gene causing 2,8-dihydroxyadenine urolithiasis". Hum. Mol. Genet. 3 (5): 817–8. doi:10.1093/hmg/3.5.817. PMID 7915931.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Adenine+phosphoribosyltransferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)