User:Hinatsuki Mikan/sandbox
 | This is a user sandbox of Hinatsuki Mikan. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Possible Articles for Editing
Physical Properties
Boiling Point
Melting Point
Structure
Spectroscopic Properties
Compared to saturated hydrocarbons, the unsaturated hydrocarbons not only contains the C-C bonds and C-H bonds, but also have C=C double bonds and C≡C triple bonds. As a result, the spectrum will also contain characteristics of these
Infrared Spectroscopy
The stretching of C=C bond will give an IR absorption peak at 1670-1600cm-1, while the bending of C=C bond absorbs between 1000-650 cm-1 wavelength. The stretching of C≡C bond absorbs 2100-2140 cm-1(monosubstituted) and 2190-2260 cm-1(disubstituted).[1] The strength of these absorption peaks varies with the place and number of the double or triple bonds.
Because of the delocalized
At the mean time, the absorption peaks of C-H and C-C bond, which are shared with the saturated hydrocarbons, also shows in the IR spectrum of unsaturated hydrocarbons.
NMR Spectroscopy
In 1H NMR spectroscopy, the hydrogen bonded to the carbon adjacent to double bonds will give a
In 13C NMR spectroscopy, compared to the saturated hydrocarbons, the double and triple bonds also deshiled the carbons, making them have low field shift. C=C double bonds usually have chemical shift of about 100-170 ppm.[4]
Chemical Properties
Combustion
Like most other hydrocarbons, unsaturated hydrocarbons can go under combustion reactions that produces carbon dioxide and water in complete combustion. The reaction equation is:
- CxHy + y+2x/2O2 → yH2O + xCO2
In the absence of oxygen, the combustion will turn into incomplete combustion and produce carbon monoxide and carbon.
The unsaturated hydrocarbons will produce incomplete combustion product more easily than saturated ones. As a result, the combustion of unsaturated hydrocarbons usually have yellow flame, different from the blue flame of the saturated ones. This indicates unsaturated hydrocarbon combustion will involve multi-step mechanisms, and the burning of carbon gives the yellow flame color.
Since unsaturated hydrocarbons have less hydrogen content, it will produce less water and decrease the flame moisture, as well as decrease the oxygen use. Acetylene(ethyne), for example, can be uesd as fuel.[5]

Compared to the single
| Number of Carbon | Substance | Type | Formula | Hcø(kJ/mol) |
|---|---|---|---|---|
| 2 | ethane | saturated | C2H6 | -1559.7 |
| ethene | unsaturated | C2H4 | -1410.8 | |
| ethyne | unsaturated | C2H2 | -1300.8 | |
| 3 | propane | saturated | CH3CH2CH3 | -2219.2 |
| propene | unsaturated | CH3CH=CH2 | -2058.1 | |
| propyne | unsaturated | CH3C≡CH | -1938.7 | |
| 4 | butane | saturated | CH3CH2CH2CH3 | -2876.5 |
| but-1-ene | unsaturated | CH2=CH-CH2CH3 | -2716.8 | |
| but-1-yne | unsaturated | CH≡C-CH2CH3 | -2596.6 |
Electrophilic Addition
The double or triple bonds that must present in unsaturated hydrocarbons provide high electron density that make the molecules become perfect spots for electrophilic addition reactions. In this kind of reaction, one
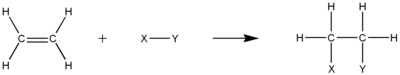
Hydrogenation
Hydrogenation is the electrophilic addition of hydrogen gas to unsaturated hydrocarbon. The result will be a more saturated hydrocarbon, but not necessarily become a saturated one. For instance, semihydrogenation of an alkyne may form an alkene. Nonetheless, the total number of
The reaction equation of hydrogenation of ethene to form ethane is:
- H2C=CH2 + H2→H3C-CH3
The hydrogenation reaction usually requires catalysts to increase its rate.
The total number of hydrogen that can be added to an unsaturated hydrocarbon depends on its degree of unsaturation. An unsaturated hydrocarbon with formula of CXHY can have 2X+2-Y hydrogen atoms at most added to it. This will make the molecule become saturated.
Halogenation
Similar as hydrogen, the heterolysis of halogen(X2) will produce a electrophilic X+ ion, after which it will be attacked by the electron on the
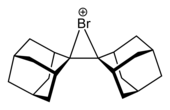
The reaction equation for bromine addition of ethene, for example, is:
- H2C=CH2 + Br2→H2CBr-CH2Br (trans)
Bromine test is used to test the saturation of hydrocarbons.[7] The test involves the addition of bromine water to the unknown hydrocarbon; If the bromine water is decolourized by the hydrocarbon, which is due to halogenation reaction, it can then be concluded that the hydrocarbon is unsaturated. If it is not decolourized, then it is saturated.
The bromine test can also determine the degree of unsaturation for unsaturated hydrocarbons. Bromine number is defined as gram of bromine able to react with 100g of product.[8] Similar as hydrogenation, the halogenation of bromine is also depend on the number of
Hydration
The
The reaction equation for hydration of ethene is:
- H2C=CH2 + H2O→H3C-CH2OH
The
The reaction equation of hydration of ethyne to form acetaldehyde is:
- HC≡CH + H2O → H2C=CH-OH
- H2C=CH-OH ⇌ H3C-CHO
Hydrohalogenation
The hydrohalogenation involves addition of H-X to unsaturated hydrocarbons. This will decrease one
The reaction equation of HBr addition to ethene is:
- H2C=CH2 + HBr→H3C-CH2Br
Polymerization
Allylic Rearrangement
Cycloaddition
References
- ^ "IR Spectrum Table & Chart". Sigma-Aldrich. Retrieved May 5, 2019.
- ^ Merlic, Craig A. "Table of IR Absorptions". Webspectra. Retrieved May 5, 2019.
- ^ Hanson, John. "Overview of Chemical Shifts in H-NMR". ups.edu. Retrieved May 5, 2019.
- ^ a b "Nuclear Magnetic Resonance (NMR) of Alkenes". Chemistry LibreTexts. April 23, 2019. Retrieved May 5, 2019.
- ^ "Acetylene The hottest and most efficient fuel gas". Linde. Retrieved May 5, 2019.
- ^ "Organic Compounds: Physical and Thermochemical Data". ucdsb.on.ca. Retrieved May 5, 2019.
- ^ R.L. Shriner, C.K.F. Hermann, T.C. Morrill, D.Y. Curtin, and R.C. Fuson (1997). The Systematic Identification of Organic Compounds. John Wiley & Sons. ISBN 0-471-59748-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Bromine Number". Hach company. Retrieved May 5, 2019.
- ^ Clark, Jim (November 2007). "THE MECHANISM FOR THE ACID CATALYSED HYDRATION OF ETHENE". Chemguide. Retrieved May 6, 2019.
- ^ "Hydration of Alkynes". Chem LibreTexts. May 2, 2019. Retrieved May 6, 2019.
- ^ "Reactions of Alkynes - Addition of HX and X₂". Chem LibreTexts. May 2, 2019. Retrieved May 6, 2019.
