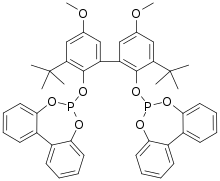
BiPhePhos
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
6,6′-[(3,3′-Di-tert-butyl-5,5′-dimethoxy-[1,1′-biphenyl]-2,2′-diyl)bis(oxy)]bis(6H-dibenzo[d,f][1,3,2]dioxaphosphepine) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C46H44O8P2 | |
| Molar mass | 786.798 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335, H412 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BiPhePhos is an organophosphorus compound that is used as a ligand in homogeneous catalysis. Classified as a diphosphite, BiPhePhos is derived from three 2,2'-biphenol groups, which constrain its shape in such a way to confer high selectivity to derived catalysts.[1] Originally described by workers at Union Carbide,[2] it has become a standard ligand in hydroformylation.[3][4]
See also
[edit]- 2,2'-Biphenylene phosphorochloridite (C12H8O2PCl) precursor to BiPhePhos.
References
[edit]- ^ Gual, Aitor; Godard, Cyril; de la Fuente, Verónica; Castillón, Sergio (2012). "Design and Synthesis of Phosphite Ligands for Homogeneous Catalysis". Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis. pp. 81–131. doi:10.1002/9781118299715.ch3. ISBN 9781118299715.
- ^ Billig, Ernst; Abatjoglou, Anthony G.; Bryant, David R. (1988). "Homogeneous Rhodium Carbonyl Compound-Phosphite Ligand Catalysts and Process for Olefin Hydroformylation". US 4769498 a 19880906 to Union Carbide.
- ^ Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized
α -olefins". Journal of the American Chemical Society. 115 (5): 2066–2068. doi:10.1021/ja00058a079. - ^ Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics. 15 (2): 835–847. doi:10.1021/OM950549K.
