Digitalose
Appearance
| |
| Names | |
|---|---|
| IUPAC name
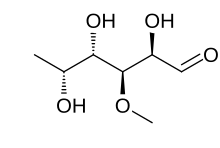
6-Deoxy-3-O-methyl-D-galactose
| |
| Other names
D-Digitalose; 6-Deoxy-3-O-methylgalactose; 3-Methyl-D-fucose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H14O5 | |
| Molar mass | 178.184 g·mol−1 |
| Melting point | 106 °C (223 °F; 379 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Digitalose is a deoxy sugar that is a component of various cardiac glycosides including thevetin and emicymarin. It was first reported in 1892 as being obtained by the hydrolysis of Digtalinum verum.[1][2] The chemical structure was first elucidated in 1943 by the German chemist Otto Schmidt.[3] Chemically, it is a methyl ether of D-fucose.
See also
[edit]- Sarmentose, a related deoxy sugar
References
[edit]- ^ a b Digitalose, Merck Index, 12th Edition, 3202
- ^ Kiliani (1892). "Ueber Digitalonsäure". Chem. Ber. 25 (1): 2116–2118. doi:10.1002/cber.189202501328.
- ^ Otto Th. Schmidt; Walter Mayer; Alfred Distelmaier (1943). "Digitalose". Naturwissenschaften. 31 (21–22): 247–248. Bibcode:1943NW.....31..247S. doi:10.1007/bf01482327.
