Disulfur dibromide
Appearance
| |
 Sulfur, S Bromine, Br | |

| |
| Names | |
|---|---|
| IUPAC names
Disulfur dibromide
Dibromodisulfane | |
| Systematic IUPAC name
Bromosulfanyl thiohypobromite | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.821 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| S2Br2 | |
| Molar mass | 223.93 g·mol−1 |
| Appearance | Orange/yellow liquid |
| Density | 2.703 g/cm3 |
| Boiling point | 46–48 °C (115–118 °F; 319–321 K) (0.1 mmHg) |
| Structure | |
| C2 | |
| 2 at sulfur atoms | |
| gauche | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
350.52 J/(mol·K)[1] |
Std enthalpy of
formation ( |
30.96 kJ/mol[1] |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| Safety data sheet (SDS) | ICSC 1661 |
| Related compounds | |
Related
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Disulfur dibromide is the inorganic compound with the formula S2Br2. It is a yellow-brown liquid that fumes in air. It is prepared by direct combination of the elements and purified by vacuum distillation.[2] The compound has no particular application, unlike the related sulfur compound disulfur dichloride.
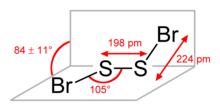
The molecular structure is Br−S−S−Br, akin to that of disulfur dichloride (S2Cl2). According to electron diffraction measurements, the angle between the Bra−S−S and S−S−Brb planes is 84° and the Br−S−S angle is 107°. The S−S distance is 198.0 pm, circa 5.0 pm shorter than for S2Cl2.[3]
References
[edit]- ^ a b Thermodynamic Properties of Inorganic Materials · Pure Substances. Part 2 _ Compounds from BeBr_g to ZrCl2_g. Springer-Verlag Berlin Heidelberg. 1999. p. 99. ISBN 978-3-540-49411-9.
- ^ F. Fehér (1963). "Dibromodisulfane". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY, NY: Academic Press. pp. 377–378.
- ^ Zysman-Colman, Eli; Harpp, David (2004). "Comparison of the Structural Properties of Compounds Containing the XSSX Moiety (X = H, Me, R, Cl, Br, F, OR)". Journal of Sulfur Chemistry. 25 (4): 291-316. doi:10.1080/17415990410001710163. S2CID 95468251.
