Plasminogen activator inhibitor-1
Plasminogen activator inhibitor-1 (PAI-1) also known as endothelial plasminogen activator inhibitor (serpin E1) is a protein that in humans is encoded by the SERPINE1 gene. Elevated PAI-1 is a risk factor for thrombosis and atherosclerosis.[5]
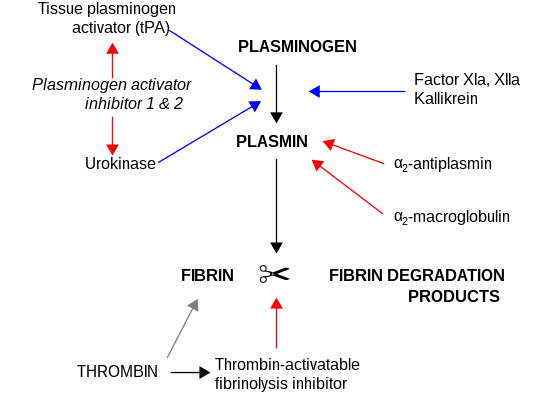
PAI-1 is a serine protease inhibitor (serpin) that functions as the principal inhibitor of tissue-type plasminogen activator (tPA) and urokinase (uPA), the activators of plasminogen and hence fibrinolysis (the physiological breakdown of blood clots). It is a serine protease inhibitor (serpin) protein (SERPINE1).
The other PAI, plasminogen activator inhibitor-2 (PAI-2) is secreted by the placenta and only present in significant amounts during pregnancy. In addition, protease nexin acts as an inhibitor of tPA and urokinase. PAI-1, however, is the main inhibitor of the plasminogen activators.
Genetics
[edit]The PAI-1 gene is SERPINE1, located on chromosome 7 (7q21.3-q22). There is a common polymorphism known as 4G/5G in the promoter region. The 5G allele is slightly less transcriptionally active than the 4G.
Function
[edit]PAI-1's main function entails the inhibition of urokinase plasminogen activator (uPA), an enzyme responsible for the cleavage of plasminogen to form plasmin. Plasmin mediates the degradation of the extracellular matrix either by itself or in conjunction with matrix metalloproteinases. In this scenario, PAI-1 inhibits uPA via active site binding, preventing the formation of plasmin. Additional inhibition is mediated by PAI-1 binding to the uPA/uPA receptor complex, resulting in the latter's degradation.[6] Thus, PAI can be said to inhibit the serine proteases tPA and uPA/urokinase, and hence is an inhibitor of fibrinolysis, the physiological process that degrades blood clots. In addition, PAI-1 inhibits the activity of matrix metalloproteinases, which play a crucial role in invasion of malignant cells through the basal lamina.
PAI-1 is mainly produced by the endothelium (cells lining blood vessels), but is also secreted by other tissue types, such as adipose tissue.
Role in disease
[edit]Congenital deficiency of PAI-1 has been reported; as fibrinolysis is not suppressed adequately, it leads to a hemorrhagic diathesis (a tendency to hemorrhage).
PAI-1 is present in increased levels in various disease states (such as a number of forms of cancer), as well as in obesity and the metabolic syndrome. It has been linked to the increased occurrence of thrombosis in patients with these conditions.
PAI-1 can induce cellular senescence.[7] PAI-1 can also be a component of the senescence-associated secretory phenotype (SASP).[8]
In inflammatory conditions in which fibrin is deposited in tissues, PAI-1 appears to play a significant role in the progression to fibrosis (pathological formation of connective tissue). Presumably, lower PAI levels would lead to less suppression of fibrinolysis and conversely a more rapid degradation of the fibrin.
Angiotensin II increases the synthesis of plasminogen activator inhibitor-1, so it accelerates the development of atherosclerosis.
Pharmacology
[edit]- Tiplaxtinin (PAI-039) is a small molecule inhibitor that is being studied for use in the attenuation of remodeling of blood vessels, a result of arterial hypertension and activation of the renin–angiotensin system.[9]
- Annonacinone is a naturally occurring PAI-1 inhibitor found in plants of the Annonaceae family.[10]
- TM5441 is another small molecule PAI-1 inhibitor used in research.[11]
Interactions
[edit]Plasminogen activator inhibitor-1 has been shown to interact with ORM1.[12]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000106366 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000037411 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Vaughan DE (August 2005). "PAI-1 and atherothrombosis". Journal of Thrombosis and Haemostasis. 3 (8): 1879–1883. doi:10.1111/j.1538-7836.2005.01420.x. PMID 16102055. S2CID 6651339.
- ^ Carter JC, Church FC (2009). "Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-
γ and plasminogen activator inhibitor-1". PPAR Research. 2009: 345320. doi:10.1155/2009/345320. PMC 2723729. PMID 19672469. - ^ Zhang M, Serna-Salas S, Damba T, Borghesan M, Demaria M, Moshage H (October 2021). "Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives". Mechanisms of Ageing and Development. 199: 111572. doi:10.1016/j.mad.2021.111572. PMID 34536446. S2CID 237524296.
- ^ Valentijn FA, Falke LL, Nguyen TQ, Goldschmeding R (March 2018). "Cellular senescence in the aging and diseased kidney". Journal of Cell Communication and Signaling. 12 (1): 69–82. doi:10.1007/s12079-017-0434-2. PMC 5842195. PMID 29260442.
- ^ Elokdah H, Abou-Gharbia M, Hennan JK, McFarlane G, Mugford CP, Krishnamurthy G, Crandall DL (July 2004). "Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization". Journal of Medicinal Chemistry. 47 (14): 3491–3494. CiteSeerX 10.1.1.661.4972. doi:10.1021/jm049766q. PMID 15214776.
- ^ Pautus S, Alami M, Adam F, Bernadat G, Lawrence DA, De Carvalho A, et al. (November 2016). "Characterization of the Annonaceous acetogenin, annonacinone, a natural product inhibitor of plasminogen activator inhibitor-1". Scientific Reports. 6: 36462. Bibcode:2016NatSR...636462P. doi:10.1038/srep36462. PMC 5120274. PMID 27876785.
- ^ Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, et al. (November 2013). "Plasminogen activator inhibitor-1 antagonist TM5441 attenuates N
ω -nitro-L-arginine methyl ester-induced hypertension and vascular senescence". Circulation. 128 (21): 2318–2324. doi:10.1161/CIRCULATIONAHA.113.003192. PMC 3933362. PMID 24092817. - ^ Boncela J, Papiewska I, Fijalkowska I, Walkowiak B, Cierniewski CS (September 2001). "Acute phase protein alpha 1-acid glycoprotein interacts with plasminogen activator inhibitor type 1 and stabilizes its inhibitory activity". The Journal of Biological Chemistry. 276 (38): 35305–35311. doi:10.1074/jbc.M104028200. PMID 11418606.
Further reading
[edit]- Mimuro J (May 1991). "[Type 1 plasminogen activator inhibitor: its role in biological reactions]". [Rinsho Ketsueki] the Japanese Journal of Clinical Hematology. 32 (5): 487–489. PMID 1870265.
- Binder BR, Christ G, Gruber F, Grubic N, Hufnagl P, Krebs M, et al. (April 2002). "Plasminogen activator inhibitor 1: physiological and pathophysiological roles". News in Physiological Sciences. 17 (2): 56–61. doi:10.1152/nips.01369.2001. PMID 11909993. S2CID 21356023.
- Eddy AA (August 2002). "Plasminogen activator inhibitor-1 and the kidney". American Journal of Physiology. Renal Physiology. 283 (2): F209–F220. doi:10.1152/ajprenal.00032.2002. PMID 12110504.
- Wang J, Li J, Liu Q (August 2005). "Association between platelet activation and fibrinolysis in acute stroke patients". Neuroscience Letters. 384 (3): 305–309. doi:10.1016/j.neulet.2005.04.090. PMID 15916851. S2CID 22979258.
- Schroeck F, Arroyo de Prada N, Sperl S, Schmitt M, Viktor M (2003). "Interaction of plasminogen activator inhibitor type-1 (PAI-1) with vitronectin (Vn): mapping the binding sites on PAI-1 and Vn". Biological Chemistry. 383 (7–8): 1143–1149. doi:10.1515/BC.2002.125. PMID 12437099. S2CID 37813055.
- Gils A, Declerck PJ (March 2004). "The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors". Thrombosis and Haemostasis. 91 (3): 425–437. doi:10.1160/TH03-12-0764. PMID 14983217. S2CID 3898268.
- Durand MK, Bødker JS, Christensen A, Dupont DM, Hansen M, Jensen JK, et al. (March 2004). "Plasminogen activator inhibitor-I and tumour growth, invasion, and metastasis". Thrombosis and Haemostasis. 91 (3): 438–449. doi:10.1160/TH03-12-0784. PMID 14983218. S2CID 3898546.
- Harbeck N, Kates RE, Gauger K, Willems A, Kiechle M, Magdolen V, Schmitt M (March 2004). "Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I: novel tumor-derived factors with a high prognostic and predictive impact in breast cancer". Thrombosis and Haemostasis. 91 (3): 450–456. doi:10.1160/TH03-12-0798. PMID 14983219. S2CID 19904733.
- Hertig A, Rondeau E (January 2004). "Plasminogen activator inhibitor type 1: the two faces of the same coin". Current Opinion in Nephrology and Hypertension. 13 (1): 39–44. doi:10.1097/00041552-200401000-00006. PMID 15090858. S2CID 30785986.
- Hoekstra T, Geleijnse JM, Schouten EG, Kluft C (May 2004). "Plasminogen activator inhibitor-type 1: its plasma determinants and relation with cardiovascular risk". Thrombosis and Haemostasis. 91 (5): 861–872. doi:10.1160/TH03-08-0546. PMID 15116245. S2CID 21576955.
- Lijnen HR (January 2005). "Pleiotropic functions of plasminogen activator inhibitor-1". Journal of Thrombosis and Haemostasis. 3 (1): 35–45. doi:10.1111/j.1538-7836.2004.00827.x. PMID 15634264. S2CID 37085650.
- De Taeye B, Smith LH, Vaughan DE (April 2005). "Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease". Current Opinion in Pharmacology. 5 (2): 149–154. doi:10.1016/j.coph.2005.01.007. PMID 15780823.
- Dellas C, Loskutoff DJ (April 2005). "Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease". Thrombosis and Haemostasis. 93 (4): 631–640. doi:10.1160/TH05-01-0033. PMID 15841306. S2CID 8937106.
- Könsgen D, Mustea A, Lichtenegger W, Sehouli J (June 2005). "[Role of PAI-1 in gynaecological malignancies]". Zentralblatt für Gynakologie. 127 (3): 125–131. doi:10.1055/s-2005-836407. PMID 15915389. S2CID 260353538.
- Hermans PW, Hazelzet JA (November 2005). "Plasminogen activator inhibitor type 1 gene polymorphism and sepsis". Clinical Infectious Diseases. 41 (Suppl 7): S453–S458. doi:10.1086/431996. PMID 16237647.
- Alessi MC, Poggi M, Juhan-Vague I (June 2007). "Plasminogen activator inhibitor-1, adipose tissue and insulin resistance". Current Opinion in Lipidology. 18 (3): 240–245. doi:10.1097/MOL.0b013e32814e6d29. PMID 17495595. S2CID 27667588.
External links
[edit]- The MEROPS online database for peptidases and their inhibitors: I04.020 Archived 2020-05-31 at the Wayback Machine
- Plasminogen+Activator+Inhibitor+1 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P05121 (Plasminogen activator inhibitor 1) at the PDBe-KB.