Chondroitin sulfate
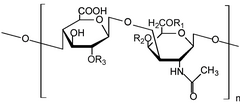

Chondroitin sulfate is a sulfated glycosaminoglycan (GAG). It is a chain of alternating sugars (N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage and provides much of its resistance to compression.[1] Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis. It is commonly extracted from shark cartilage.
Terminology
[change | change source]Chondroitin sulfate was originally isolated well before chemists learned its structure. So, its name changed over time.[2] Early researchers identified different types of the substance with letters.
| Letter identification | Site of sulfation | Systematic name |
| Chondroitin sulfate A | carbon 4 of the N-acetylgalactosamine (GalNAc) sugar | chondroitin-4-sulfate |
| Chondroitin sulfate C | carbon 6 of the GalNAc sugar | chondroitin-6-sulfate |
| Chondroitin sulfate D | carbon 2 of the glucuronic acid and 6 of the GalNAc sugar | chondroitin-2,6-sulfate |
| Chondroitin sulfate E | carbons 4 and 6 of the GalNAc sugar | chondroitin-4,6-sulfate |
"Chondroitin sulfate B" is an old name for dermatan sulfate, and it is no longer classified as a form of chondroitin sulfate.[3]
Chondroitin, without the "sulfate", has been used to describe a type with little or no sulfation.[4] However, this distinction is not used by all.
The name "chondroitin sulfate" sounds like a salt with a sulfate counter-anion. This is not the case. The sulfate is attached to the sugar with a covalent bond. Since the molecule has multiple negative charges at physiological pH, a cation is present in salts of chondroitin sulfate. Commercial preparations of chondroitin sulfate typically are the sodium salt. Barnhill et al. have suggested that all such preparations of chondroitin sulfate be referred to as "sodium chondroitin" regardless of their sulfation status.[5]
Structure
[change | change source]Chondroitin sulfate chains are unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid (GlcA) and N-acetyl-D-galactosamine (GalNAc). Some GlcA residues are epimerized into L-iduronic acid (IdoA); the resulting disaccharide is then referred to as dermatan sulfate.
Protein attachment
[change | change source]Chondroitin sulfate chains are linked to hydroxyl groups on serine residues of certain proteins. Exactly how proteins are selected for attachment of glycosaminoglycans is not understood. Glycosylated serines are often followed by a glycine and have neighboring acidic residues. But this does not always predict glycosylation.
Attachment of the GAG chain begins with four monosaccharides in a fixed pattern: Xyl - Gal - Gal - GlcA. Each sugar is attached by a specific enzyme, allowing for multiple levels of control over GAG synthesis. Xylose begins to be attached to proteins in the endoplasmic reticulum, while the rest of the sugars are attached in the Golgi apparatus.[6]
Sulfation
[change | change source]Each monosaccharide may be left unsulfated, sulfated once, or sulfated twice. In the most common case, the hydroxyls of the 4 and 6 positions of the N-acetyl-galactosamine are sulfated, with some chains having the 2 position of glucuronic acid. Sulfation is mediated by specific sulfotransferases. Sulfation in these different positions gives specific biological activities to chondroitin GAG chains.
Function
[change | change source]Chondroitin's functions depend largely on the properties of the overall proteoglycan of which it is a part. These functions can be broadly divided into structural and regulatory roles. Some proteoglycans have both structural and regulatory roles (see versican).
Structural
[change | change source]Chondroitin sulfate is a major component of extracellular matrix, and is important in maintaining the structural integrity of tissue. This function is typical of the large aggregating proteoglycans: aggrecan, versican, brevican, and neurocan, collectively termed the lecticans.
As part of aggrecan, chondroitin sulfate is a major component of cartilage. The tightly packed and highly charged sulfate groups of chondroitin sulfate generate electrostatic repulsion that provides much of the resistance of cartilage to compression. Loss of chondroitin sulfate from the cartilage is a major cause of osteoarthritis.
Regulatory
[change | change source]Chondroitin sulfate readily interacts with proteins in the extracellular matrix due to its negative charges. These interactions are important for regulating many cellular activities. The lecticans are a major part of the brain extracellular matrix, where the chondroitin sugar chains function to stabilize normal brain synapses as part of perineuronal nets. The levels of chondroitin sulfate proteoglycans are vastly increased after injury to the central nervous system where they act to prevent regeneration of damaged nerve endings. Although these functions are not as well characterized as those of heparan sulfate, new roles continue to be discovered for the chondroitin sulfate proteoglycans.
In cortical development, chondroitin sulfate is expressed by the Sub Plate and acts as a stop signal for neurons migrating from the Ventricular Zone. Neurons stopping here may then be programmed for further migration to specific layers in the cortical plate.
Medical use
[change | change source]Chondroitin is in dietary supplements used as an alternative medicine to treat osteoarthritis and also approved and regulated as a symptomatic slow-acting drug for this disease (SYSADOA) in Europe and some other countries.[7] It is commonly sold together with glucosamine. Chondroitin and glucosamine are also used in veterinary medicine.[8]
References
[change | change source]- ↑ Baeurle SA, Kiselev MG, Makarova ES, Nogovitsin EA (2009). "Effect of the counterion behavior on the frictional–compressive properties of chondroitin sulfate solutions". Polymer. 50 (7): 1805–1813. doi:10.1016/j.polymer.2009.01.066.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ P. A. Levene and F. B. La Forge (1913). "On Chondroitin Sulphuric Acid". J. Biol. Chem. 15: 69–79. doi:10.1016/S0021-9258(18)88542-8. Free PDF online Archived 2008-11-05 at the Wayback Machine
- ↑ MeSH Chondroitin+sulfates
- ↑ Davidson EA, Meyer K (1954). "Chondroitin, a new mucopolysaccharide". J Biol Chem. 211 (2): 605–11. doi:10.1016/S0021-9258(18)71150-2. PMID 13221568. Free PDF online Archived 2008-09-22 at the Wayback Machine
- ↑ Barnhill JG, Fye CL, Williams DW, Reda DJ, Harris CL, Clegg DO (2006). "Chondroitin product selection for the glucosamine/chondroitin arthritis intervention trial". J Am Pharm Assoc (Wash DC). 46 (1): 14–24. doi:10.1331/154434506775268616. PMID 16529337.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Silbert JE, Sugumaran G (2002). "Biosynthesis of chondroitin/dermatan sulfate". IUBMB Life. 54 (4): 177–86. doi:10.1080/15216540214923. PMID 12512856. S2CID 11564009.
- ↑ Jordan KM, Recommendations Arden NK. EULAR (2003). "an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)". Ann Rheum Dis. 62 (12): 1145–1155. doi:10.1136/ard.2003.011742. PMC 1754382. PMID 14644851.
- ↑ Forsyth R, Brigden C, Northrop A (2006). "Double blind investigation of the effects of oral supplementation of combined glucosamine hydrochloride (GHCL) and chondroitin sulfate (CS) on stride characteristics of veteran horses". Equine Veterinary Journal. Supplement. 38 (36): 622–5. doi:10.1111/j.2042-3306.2006.tb05615.x. PMID 17402494. S2CID 3037365.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Other websites
[change | change source]- General Glucosamine and Chondroitin Sulfate information Archived 2006-11-05 at the Wayback Machine at Arthritis Foundation
- "Product Review: Joint Supplements (Glucosamine, Chondroitin, and MSM)" Archived 2008-08-20 at the Wayback Machine Summary of a Consumer Labs test of the actual composition of these supplements at consumerlabs.com
- "Glucosamine/Chondroitin Products Not Measuring Up", News report of the analysis of commercial supplements by Adebowale et al. at WebMD
- "Chondroitin Sulfate Manufacturing and Risk of Mad Cow Disease" by Winston Wicomb, Ph.D., September 24, 2002. Information on methods for extraction of chondrotin sulfate from cow trachea, at the Stone Clinic of San Francisco, at stoneclinic.com
- "Testing Status: Chondroitin Sulfate M030009", A thorough review of available information on the use of chondroitin sulfate in humans from the National Toxicology Program at National Institute of Environmental Health Sciences
- Chondroitin Sulfate, summary of information on the use of chondroitin sulfate from the publishers of the Physicians' Desk Reference.
- "Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT)," ClinicalTrials.gov information on the purpose, design, and analysis of the study at clinicaltrials.gov
- "NIH News: Efficacy of Glucosamine and Chondroitin Sulfate May Depend on Level of Osteoarthritis Pain", Wednesday, February 22, 2006 at National Institutes of Health
