Carbon Dioxide Removal Program
![]()
Advancing a diverse set of CDR approaches to directly remove CO2 emissions from the atmosphere, to aid in facilitating gigaton-scale removal by 2050, with an emphasis on rigorous analysis of life cycle impacts and a deep commitment to environmental justice
Carbon dioxide removal (CDR) is a crosscutting research, development, and demonstration (RD&D) area for the U.S. Department of Energy (DOE). The Office of Fossil Energy and Carbon Management (FECM), the Office of Energy Efficiency and Renewable Energy (EERE), the Office of Science, and the Advanced Research Projects Agency-Energy (ARPA-E) have existing efforts in CDR. These coordinated efforts contribute to Carbon Negative Shot, the third target within DOE’s Energy Earthshots Initiative, which requires multiple CDR approaches be enabled at scale to achieve the net-zero emissions in the United States by 2050. DOE is developing a wide array of CDR approaches, such as direct air capture (DAC) with durable storage, biomass carbon removal and storage (BiCRS), enhanced mineralization, ocean-based CDR, soil carbon storage, and afforestation/reforestation. Together, these approaches will help achieve gigaton-scale removal by 2050 and support the United States in achieving ambitious goals for a greenhouse gas (GHG)-neutral economy by 2050, a carbon-pollution-free power sector by 2035, and a 50% reduction from 2005 levels in economy-wide net GHG pollution by 2030. The benefits, risks, and sustainable-scale potential for various CDR approaches are discussed in a report released in 2019 by the National Academies of Sciences, Engineering, and Medicine—and co-funded by FECM’s Carbon Capture Program—titled “Negative Emissions Technologies and Reliable Sequestration: A Research Agenda.”i
DOE’s FECM has adopted a comprehensive, multi-pronged approach for carbon management that involves the coupling of carbon capture methods (i.e., CDR technologies co-located with low-carbon energy sources; and point source capture for fossil fuel-based power generation and industrial sources) with long-duration carbon storage or CO2 utilization/conversion into long-lasting products. The National Energy Technology Laboratory’s (NETL) CDR Program is fostering research and development (R&D) focused on DAC, with emerging research in the areas of BiCRS, enhanced mineralization, and ocean-based and terrestrial CDR approaches to remove CO2 that has accumulated in the atmosphere. The captured CO2 will then be securely stored in geologic, bio-based, and ocean reservoirs, or in value-added products, resulting in negative emissions (i.e., more carbon is removed from the atmosphere than generated). The CDR Program also emphasizes a robust analysis of life cycle impacts and a deep commitment to environmental justice. Projects range from conceptual engineering and materials design at laboratory and bench scale (Technology Readiness Level [TRL] 2–5) to large-scale testing and front-end engineering and design (FEED) studies (TRL 6–7) to lower both capital and operating costs and improve the economics of CDR.
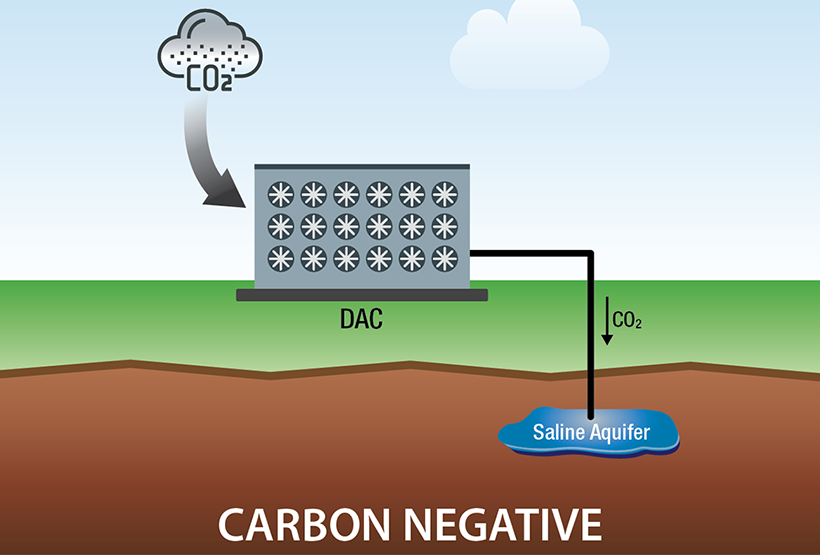
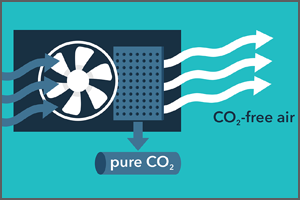
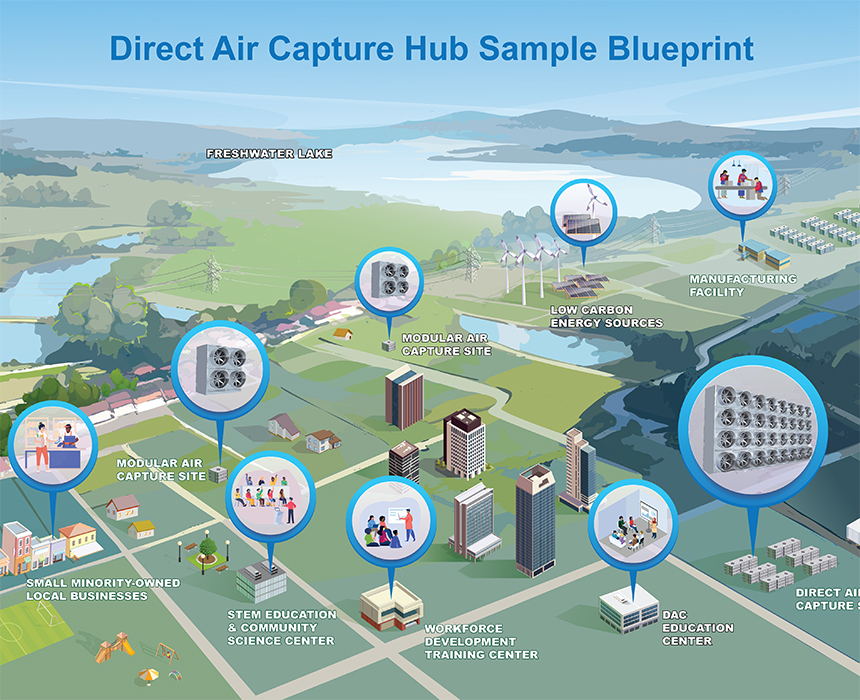
Direct Air Capture
The CDR Program is advancing intentional technological interventions for the purpose of creating negative emissions through durable storage. While these approaches differ significantly in their energy use, land use, and social implications, they will all require substantial R&D efforts to enable a responsible and efficient CDR industry that is responsive to the climate crisis. Challenges include the dilute concentration of CO2 in the atmosphere, large water and land use requirements, carbon life cycle effectiveness, low-carbon energy integration, low-cost durable storage, and impacts of process pressure drop on overall energy use and system cost. The development of robust life cycle analysis and monitoring, reporting and verification (MRV) methods are critical to ensuring effective and permanent CO2 removal for all CDR approaches. Note that CDR does NOT refer to existing systems that remove CO2 from the atmosphere; for example, CDR does NOT include CO2 removal through existing trees or by planting new trees to offset wildfire losses.

Biomass Carbon Removal and Storage
Biomass carbon removal and storage (BiCRS) is a CDR approach involving the use of biomass (e.g., algae, municipal waste, agricultural and wood residues) to remove CO2 from the atmosphere and store it underground or in long-lived products, without affecting food security, rural livelihoods, biodiversity conservation, and other important valuesii. Carbon dioxide is produced from the combustion, gasification, pyrolysis, or other conversion of biomass to generate electricity or produce hydrogen, and the resulting CO2 emissions are captured and then stored in a manner that prevents entry into the atmosphere. Challenges associated with BiCRS include water and land requirements; cultivating, transporting, and processing microalgae at large scale; and difficulties in measuring, monitoring, and crediting carbon removal.
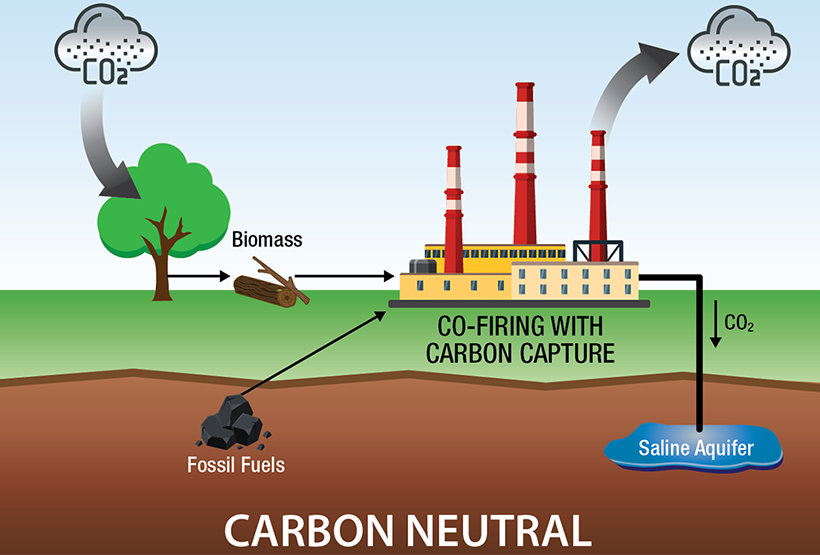
One common type of BiCRS is bioenergy with carbon capture and storage (BECCS), which combines energy production using plant biomass to produce electricity, liquid fuels, and/or heat with the capture and storage of the CO2 produced when using the bioenergy. Because the biomass draws CO2 from the atmosphere as it grows, BECCS can be considered a negative-emissions technology. The co-firing of biomass (e.g., switchgrass, wood pellets, corn stover) with fossil fuels reduces the CO2 life cycle footprint of existing power plants. However, large-scale implementation of BECCS is constrained by land availability for competing uses (i.e., food production) and low power plant efficiency.

Enhanced Mineralization
Enhanced mineralization (also known as enhanced weathering or accelerated weathering) is a CDR approach that uses technologies or modified land-use approaches to accelerate the decomposition of calcium- and magnesium-rich silicate rocks, which chemically react with CO2 to form solid carbonate minerals, resulting in the removal of CO2 from the atmosphere. There are several types of mineralization processes: in situ (e.g., CO2 reactions in geologic formations underground), ex situ (e.g., CO2 reactions that involve extraction, transport, and grinding of minerals), and surficial (e.g., CO2 reactions with minerals distributed across land or coastal areas). Enhanced mineralization can help stabilize and permanently store waste from certain industries (e.g., mine tailings). Research is needed to assess this potential across different waste streams and to ensure that real benefits in environmental remediation are seen by local communities.
Marine Carbon Dioxide Removal
Marine CDR (mCDR) is a CDR approach that removes CO2 from the atmosphere and upper ocean and securely stores the excess carbon either in marine or geological reservoirs. The ocean acts as a large natural reservoir for CO2, removing a substantial fraction of the excess atmospheric CO2; however, due to several physical, geochemical, and biological processes that influence the exchange between air and oceans, an excess of CO2 in the oceans can occur, leading to changes in seawater chemistry- (i.e., ocean acidification) and negative impacts on ecosystems. mCDR approaches have the potential to promote atmospheric CO2 uptake by the ocean while mitigating ocean acidification, and thus contribute to the restoration of ecosystems.
A report released in 2021 by the National Academies of Sciences, Engineering, and Medicine titled “A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration” discusses the benefits, risks, and potential of mCDR as well as six ecosystem- and technological-based approaches to be considered.iii The approaches span a wide range of biotic (i.e., nutrient fertilization, artificial up- and down-welling, seaweed cultivation, and ecosystem recovery) and abiotic (e.g., electrochemical engineering processes and alkalinity enhancement) methodologies. The CDR Program is focused on the advancement of abiotic approaches. Electrochemical methods consume electricity to drive the separation of acid and base solutions to extract CO2 from seawater in the form of a gas or as mineral carbonates. Ocean alkalinity enhancement is the chemical alteration of seawater chemistry via addition of alkalinity through various mechanisms (e.g., adding large amounts of pulverized silicate or carbonate rock) thereby “locking” CO2 into other forms of dissolved inorganic carbon (DIC), which is expected to promote atmospheric CO2 influx into the ocean.
Key Technology Areas
Solvents

Solvent-based CO2 capture involves chemical or physical absorption of CO2 from air or gas into a liquid carrier. The absorption liquid is regenerated by increasing its temperature or reducing its pressure to break the absorbent-CO2 bond. Carbon dioxide capture from air is possible with commercially available chemical solvent-based systems; however, these systems require significant amounts of energy for regeneration. R&D objectives include advanced solvents (e.g., water-lean solvents, phase-change solvents, high-performance functionalized solvents) for extraction from dilute CO2 sources, which also have a lower regeneration energy requirement than existing amine systems, combined with high CO2 absorption capacity. System advancements include process intensification techniques, methods to mitigate aerosol formation and corrosion, and heat integration approaches.
Sorbents

Sorbent-based CO2 capture involves the chemical or physical adsorption of CO2 using a solid sorbent. Like solvents, solid sorbents are usually regenerated by increasing temperature or reducing pressure to release the captured CO2. In addition, sorbent-based capture technologies exhibit greater modularity than solvent-based systems, providing an advantage for large-scale operation. Optimizing the long-term stability and heat transfer properties of sorbents are some of the major challenges, which can be addressed through sorbent washcoating techniques. R&D objectives include novel sorbent support structures, such as fibers, monoliths, and laminates, and low-cost durable sorbents that have high selectivity for CO2 from dilute sources, high CO2 adsorption capacity, and resistance to oxidation. System advancements include sorbent process intensification techniques, novel reactor designs, and enhanced process configurations, such as novel stationary or rotating beds for CO2 adsorption and desorption.
Membranes

Membrane-based CO2 capture uses permeable or semi-permeable materials that allow for the selective transport and separation of CO2 from air or gas. Membrane processes offer potential advantages when applied to CO2 capture, including no hazardous chemical storage, handling, disposal, or emissions issues; simple passive operation; a reduced process footprint; and modular nature. The challenge with membranes is the separation of CO2 from very low partial pressure of CO2 in air. R&D objectives include development of low-cost, durable membranes (e.g., polymeric membranes, mixed matrix membranes, sub-ambient temperature membranes) that have improved permeability and selectivity for CO2, especially when nitrogen and oxygen are in excess. Process enhancements for membrane-based capture systems include low pressure drop membrane modules.
Electrochemical Novel Concepts
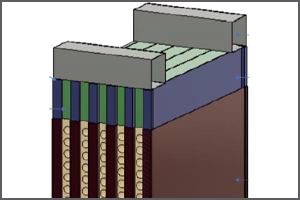
Electrochemical-based CO2 capture uses pH swing cycle that changes conditions between basic and acidic to capture and release CO2. Electrochemical processes offer potential advantages by having a lower regeneration energy requirement than existing amine-based systems. Additionally, hydrogen is produced as a byproduct of the electrochemical process, which can be used to produce hydrocarbons and valuable chemical products. Electrochemical processes can be easily integrated with renewable power sources to reduce the cost and improve the efficiency of CO2 capture systems. The challenge with electrochemical processes involves the design of electrochemical cell and gas-liquid contactors for large-scale applications. R&D objectives include the development of improved electrode materials, membrane contactors, and scaling up systems for single-stack and/or multi-stack modular configurations.