Β -Pinene: Difference between revisions
No edit summary |
Citation bot (talk | contribs) Alter: edition, url. URLs might have been anonymized. Add: authors 1-1. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Jay8g | Category:CS1 errors: extra text: edition | #UCB_Category 2/2 |
||
| (45 intermediate revisions by 24 users not shown) | |||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE: |
{{DISPLAYTITLE: |
||
{{chembox |
{{chembox |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| Line 8: | Line 8: | ||
| ImageFileL1 = (1S)-(-)-beta-pinene-2D-projected-skeletal.png |
| ImageFileL1 = (1S)-(-)-beta-pinene-2D-projected-skeletal.png |
||
| ImageFileR1 = (1S)-(−)-beta-pinene-from-xtal-3D-balls.png |
| ImageFileR1 = (1S)-(−)-beta-pinene-from-xtal-3D-balls.png |
||
| |
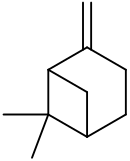
| IUPACName = 6,6-Dimethyl-2-methylidenebicyclo[3.1.1]heptane<br />Pin-2(10)-ene |
||
| OtherNames = 6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane<br />2(10)-Pinene<br />Nopinene<br />Pseudopinene |
| OtherNames = 6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane<br />2(10)-Pinene<br />Nopinene<br />Pseudopinene |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| DrugBank = DB15574 |
|||
| EINECS = 204-872-5 |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| KEGG = C09882 |
| KEGG = C09882 |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| InChI = 1/C10H16/c1-7-4-5-8-6-9(7)10(8,2)3/h8-9H,1,4-6H2,2-3H3 |
| InChI = 1/C10H16/c1-7-4-5-8-6-9(7)10(8,2)3/h8-9H,1,4-6H2,2-3H3 |
||
| InChIKey = WTARULDDTDQWMU-UHFFFAOYAW |
| InChIKey = WTARULDDTDQWMU-UHFFFAOYAW |
||
| ⚫ | |||
| ⚫ | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C10H16/c1-7-4-5-8-6-9(7)10(8,2)3/h8-9H,1,4-6H2,2-3H3 |
| StdInChI = 1S/C10H16/c1-7-4-5-8-6-9(7)10(8,2)3/h8-9H,1,4-6H2,2-3H3 |
||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = WTARULDDTDQWMU-UHFFFAOYSA-N |
| StdInChIKey = WTARULDDTDQWMU-UHFFFAOYSA-N |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| SMILES = C1(=C)C2CC(CC1)C2(C)C |
| SMILES = C1(=C)C2CC(CC1)C2(C)C |
||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| Line 36: | Line 38: | ||
| Appearance = Colorless liquid |
| Appearance = Colorless liquid |
||
| Density = 0.872 g/mL |
| Density = 0.872 g/mL |
||
| |
| MeltingPtK = 211.61 |
||
| MeltingPt_ref = <ref>{{ |
| MeltingPt_ref = <ref name="auto">{{cite web | url = http://webbook.nist.gov/cgi/cbook.cgi?Name=beta-pinene&Units=SI&cTG=on&cIR=on&cTC=on&cTZ=on&cTP=on&cMS=on&cTR=on&cUV=on&cIE=on&cGC=on&cIC=on&cES=on&cDI=on&cSO=on | title = |
||
| |
| BoilingPtK = 438-440 |
||
| BoilingPt_ref = <ref>{{ |
| BoilingPt_ref = <ref>{{cite web | url = https://www.sigmaaldrich.com/catalog/product/aldrich/402753?lang=pt®ion=BR | title = (−)- |
||
| Solubility = |
| Solubility = |
||
}} |
}} |
||
|Section3={{Chembox |
|Section3={{Chembox Thermochemistry |
||
| DeltaHc = {{val|-6214.1|2.9|u=kJ/mol}}<ref name="auto"/> |
|||
}} |
|||
|Section4={{Chembox Hazards |
|||
| MainHazards = |
| MainHazards = |
||
| FlashPtC = 36 |
| FlashPtC = 36 |
||
| Line 49: | Line 54: | ||
| NFPA-F = 3 |
| NFPA-F = 3 |
||
| NFPA-R = 0 |
| NFPA-R = 0 |
||
| |
| GHSPictograms = {{GHS02}}{{GHS07}}{{GHS08}}{{GHS09}} |
||
| GHSSignalWord = Danger |
|||
| SPhrases = {{S26}} {{S36}} |
|||
| HPhrases = {{H-phrases|226|304|315|317|410}} |
|||
| PPhrases = {{P-phrases|210|233|240|241|242|243|261|264|272|273|280|301+310|302+352|303+361+353|321|331|332+313|333+313|362|363|370+378|391|403+235|405|501}} |
|||
}} |
}} |
||
}} |
}} |
||
''' |
''' |
||
== Sources == |
|||
== Plants that contain |
|||
Many plants from many botanical families contain the compound, including: |
Many plants from many botanical families contain the compound, including: |
||
* ''[[Cuminum cyminum]]''<ref name=li> |
* ''[[Cuminum cyminum]]''<ref name=li>{{cite journal | doi = 10.1002/ffj.1302| title = Chemical composition of the essential oil of ''Cuminum'' cyminum L. From China| year = 2004| last1 = Li| first1 = Rong| last2 = Jiang| first2 = Zi-Tao| journal = Flavour and Fragrance Journal| volume = 19| issue = 4| pages = 311–313}}</ref><ref>{{cite journal | pmid = 19576388 | year = 2009 | last1 = Wang | first1 = L. | last2 = Wang | first2 = Z. | last3 = Zhang | first3 = H. | last4 = Li | first4 = X. | last5 = Zhang | first5 = H. | title = Ultrasonic nebulization extraction coupled with headspace single drop microextraction and gas chromatography-mass spectrometry for analysis of the essential oil in Cuminum cyminum L | journal = Analytica Chimica Acta | volume = 647 | issue = 1 | pages = 72–7 | doi = 10.1016/j.aca.2009.05.030 }}</ref> |
||
*''[[Humulus lupulus]]''<ref>Tinseth, G. [http://morebeer.com/brewingtechniques/library/backissues/issue2.1/tinseth.html The Essential Oil of Hops: Hop Aroma and Flavor in Hops and Beer.] ''Brewing Techniques'' January/February 1994. Accessed July 21, 2010.</ref> |
*''[[Humulus lupulus]]''<ref>Tinseth, G. [http://morebeer.com/brewingtechniques/library/backissues/issue2.1/tinseth.html The Essential Oil of Hops: Hop Aroma and Flavor in Hops and Beer.] {{Webarchive|url=https://web.archive.org/web/20131111102913/http://morebeer.com/brewingtechniques/library/backissues/issue2.1/tinseth.html |date=2013-11-11 }} ''Brewing Techniques'' January/February 1994. Accessed July 21, 2010.</ref> |
||
* ''[[Pinus pinaster]]''<ref name=herm/> |
* ''[[Pinus pinaster]]''<ref name=herm/> |
||
* ''[[Clausena anisata]]'' |
* ''[[Clausena anisata]]'' |
||
* ''[[Cannabis sativa]]''<ref>{{Cite journal|last=Hillig|first=Karl W|date=October 2004|title=A chemotaxonomic analysis of terpenoid variation in Cannabis|journal=Biochemical Systematics and Ecology|volume=32|issue=10|pages=875–891|doi=10.1016/j.bse.2004.04.004|issn=0305-1978}}</ref> |
|||
* ''[[Cannabis sativa]]'' |
|||
* ''[[Black pepper|Piper nigrum]]''<ref name=":0">{{Cite book |last=Santana de Oliveira |first=Mozaniel |title=Essential oils: applications and trends in food science and technology |date=2022 |publisher=Springer |isbn=978-3-030-99476-1 |location=Cham, Switzerland}}</ref> |
|||
* ''[[Cannabis indica]]'' |
|||
* ''[[Myristica fragrans|Myristica fragans .]]''<ref name=":0" /> |
|||
* ''[[Key lime|Citrus aurantiifolia]]''<ref name=":0" /> |
|||
* ''[[Pistacia lentiscus]]''<ref name=":0" /> |
|||
The clear compund is produced by [[distillation]] of [[Turpentine|turpentine oils]].<ref name=":1">{{Cite book |last1=Surburg |first1=Horst |title=Common fragrance and flavor materials: preparation, properties and uses |last2=Panten |first2=Johannes |date=2016 |publisher=Wiley-VCH Verlag GmbH & Co. KGaA |isbn=978-3-527-33160-4 |edition=6. completely revised and updated |location=Weinheim}}</ref> |
|||
== |
==Uses== |
||
{{expand section|date=October 2023}} |
|||
* [[Lemon]] |
|||
==References== |
==References== |
||
| Line 77: | Line 89: | ||
[[Category:Flavors]] |
[[Category:Flavors]] |
||
[[Category:Perfume ingredients]] |
[[Category:Perfume ingredients]] |
||
[[Category: |
[[Category:Vinylidene compounds]] |
||
[[Category:Monoterpenes]] |
[[Category:Monoterpenes]] |
||
[[Category:Bicyclic compounds]] |
[[Category:Bicyclic compounds]] |
||
Latest revision as of 22:13, 9 May 2024
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
6,6-Dimethyl-2-methylidenebicyclo[3.1.1]heptane
Pin-2(10)-ene | |||
| Other names
6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane
2(10)-Pinene Nopinene Pseudopinene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.004.430 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.872 g/mL | ||
| Melting point | −61.54 °C; −78.77 °F; 211.61 K[1] | ||
| Boiling point | 165–167 °C; 329–332 °F; 438–440 K[2] | ||
| Thermochemistry | |||
Std enthalpy of
combustion ( |
−6214.1±2.9 kJ/mol[1] | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H226, H304, H315, H317, H410 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P272, P273, P280, P301+P310, P302+P352, P303+P361+P353, P321, P331, P332+P313, P333+P313, P362, P363, P370+P378, P391, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 36 °C (97 °F; 309 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sources[edit]
Many plants from many botanical families contain the compound, including:
- Cuminum cyminum[5][6]
- Humulus lupulus[7]
- Pinus pinaster[4]
- Clausena anisata
- Cannabis sativa[8]
- Piper nigrum[9]
- Myristica fragans .[9]
- Citrus aurantiifolia[9]
- Pistacia lentiscus[9]
The clear compund is produced by distillation of turpentine oils.[10]
Uses[edit]
This section needs expansion. You can help by adding to it. (October 2023) |
References[edit]
- ^ a b "
β -Pinene". National Institute of Standards and Technology. Retrieved January 29, 2018. - ^ "(−)-
β -Pinene". Sigma-Aldrich. Retrieved January 29, 2018. - ^ Geron, C., et al. (2000). A review and synthesis of monoterpene speciation from forests in the United States. Atmospheric Environment 34(11), 1761-81.
- ^ a b Neuenschwander, U.; Meier, E.; Hermans, I. (2011). "Peculiarities of
β -pinene autoxidation". ChemSusChem. 4 (11): 1613–21. doi:10.1002/cssc.201100266. PMID 21901836. - ^ Li, Rong; Jiang, Zi-Tao (2004). "Chemical composition of the essential oil of Cuminum cyminum L. From China". Flavour and Fragrance Journal. 19 (4): 311–313. doi:10.1002/ffj.1302.
- ^ Wang, L.; Wang, Z.; Zhang, H.; Li, X.; Zhang, H. (2009). "Ultrasonic nebulization extraction coupled with headspace single drop microextraction and gas chromatography-mass spectrometry for analysis of the essential oil in Cuminum cyminum L". Analytica Chimica Acta. 647 (1): 72–7. doi:10.1016/j.aca.2009.05.030. PMID 19576388.
- ^ Tinseth, G. The Essential Oil of Hops: Hop Aroma and Flavor in Hops and Beer. Archived 2013-11-11 at the Wayback Machine Brewing Techniques January/February 1994. Accessed July 21, 2010.
- ^ Hillig, Karl W (October 2004). "A chemotaxonomic analysis of terpenoid variation in Cannabis". Biochemical Systematics and Ecology. 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004. ISSN 0305-1978.
- ^ a b c d Santana de Oliveira, Mozaniel (2022). Essential oils: applications and trends in food science and technology. Cham, Switzerland: Springer. ISBN 978-3-030-99476-1.
- ^ a b Surburg, Horst; Panten, Johannes (2016). Common fragrance and flavor materials: preparation, properties and uses (6. completely revised and updated ed.). Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA. ISBN 978-3-527-33160-4.
- ^ Opdyke, D. L. J. (2013-10-22). Monographs on Fragrance Raw Materials: A Collection of Monographs Originally Appearing in Food and Cosmetics Toxicology. Elsevier. ISBN 978-1-4831-4797-0.
- ^ Mattiello, Joseph J. (1945). Protective and Decorative Coatings. U.S. Government Printing Office.
- ^ Opdyke, D. L. J. (2013-10-22). Monographs on Fragrance Raw Materials: A Collection of Monographs Originally Appearing in Food and Cosmetics Toxicology. Elsevier. ISBN 978-1-4831-4797-0.



