Β -Pinene: Difference between revisions
Content deleted Content added
No edit summary |
No edit summary |
||
| Line 39: | Line 39: | ||
| MeltingPt_ref = <ref>{{Cite book | title =http://webbook.nist.gov/cgi/cbook.cgi?Name=beta-pinene&Units=SI&cTG=on&cIR=on&cTC=on&cTZ=on&cTP=on&cMS=on&cTR=on&cUV=on&cIE=on&cGC=on&cIC=on&cES=on&cDI=on&cSO=on visited on 01/29/2018 }}</ref> |
| MeltingPt_ref = <ref>{{Cite book | title =http://webbook.nist.gov/cgi/cbook.cgi?Name=beta-pinene&Units=SI&cTG=on&cIR=on&cTC=on&cTZ=on&cTP=on&cMS=on&cTR=on&cUV=on&cIE=on&cGC=on&cIC=on&cES=on&cDI=on&cSO=on visited on 01/29/2018 }}</ref> |
||
| BoilingPt = 438-440 K |
| BoilingPt = 438-440 K |
||
| BoilingPt_ref = {{Cite book | title =https://www.sigmaaldrich.com/catalog/product/aldrich/402753?lang=pt®ion=BR visited on 01/29/2018 }}</ref> |
| BoilingPt_ref = <ref>{{Cite book | title =https://www.sigmaaldrich.com/catalog/product/aldrich/402753?lang=pt®ion=BR visited on 01/29/2018 }}</ref> |
||
| Solubility = |
| Solubility = |
||
}} |
}} |
||
Revision as of 16:43, 29 January 2018
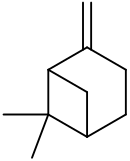
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
6,6-Dimethyl-2-methylidenebicyclo[3.1.1]heptane | |||
| Other names
6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptane
2(10)-Pinene Nopinene Pseudopinene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.430 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.872 g/mL | ||
| Melting point | 211.61 K[1] | ||
| Boiling point | 438-440 K[2] | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 36 °C (97 °F; 309 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
beta-Pinene (
This is one of the most abundant compounds released by forest trees.[3] If oxidized in air, the allylic products of the pinocarveol and myrtenol family prevail.[4]
Plants that contain β -pinene
Many plants from many botanical families contain the compound, including:
- Cuminum cyminum[5][6]
- Humulus lupulus[7]
- Pinus pinaster[4]
- Clausena anisata
- Cannabis sativa
- Cannabis indica
See also
References
- ^ http://webbook.nist.gov/cgi/cbook.cgi?Name=beta-pinene&Units=SI&cTG=on&cIR=on&cTC=on&cTZ=on&cTP=on&cMS=on&cTR=on&cUV=on&cIE=on&cGC=on&cIC=on&cES=on&cDI=on&cSO=on visited on 01/29/2018.
{{cite book}}: External link in|title= - ^ https://www.sigmaaldrich.com/catalog/product/aldrich/402753?lang=pt®ion=BR visited on 01/29/2018.
{{cite book}}: External link in|title= - ^ Geron, C., et al. (2000). A review and synthesis of monoterpene speciation from forests in the United States. Atmospheric Environment 34(11), 1761-81.
- ^ a b Neuenschwander, U., et al. (2011). Peculiarities of
β -pinene autoxidation. ChemSusChem 4(11), 1613-21. - ^ Li, R. and Z. T. Jiang. (2004). Chemical composition of the essential oil of Cuminum cyminum L. from China. Flavour and Fragrance Journal 19(4), 311-13.
- ^ Wang, L., et al. (2009). Ultrasonic nebulization extraction coupled with headspace single drop microextraction and gas chromatography-mass spectrometry for analysis of the essential oil in Cuminum cyminum L. Analytica Chimica Acta 647(1), 72-77.
- ^ Tinseth, G. The Essential Oil of Hops: Hop Aroma and Flavor in Hops and Beer. Brewing Techniques January/February 1994. Accessed July 21, 2010.



