Beta-lactam antibiotics
| Drug class | |
 Core structure of penicillins (top) and cephalosporins (bottom) the 2 most common groups of | |
| Class identifiers | |
| Use | Bacterial infection |
| ATC code | J01C |
| Biological target | Penicillin binding protein |
| External links | |
| MeSH | D047090 |
| Legal status | |
| In Wikidata | |
Bacteria often develop resistance to
Medical use
[edit]In uninflamed (normal) brain meninges, the penetration of beta-lactam antibiotics is low, at 0.15 of
Adverse effects
[edit]Adverse drug reactions
[edit]Common adverse drug reactions for the
Infrequent adverse effects include fever, vomiting, erythema, dermatitis, angioedema, pseudomembranous colitis.[8]
Pain and inflammation at the injection site is also common for parenterally administered
Allergy/hypersensitivity
[edit]Immunologically mediated adverse reactions to any
Nevertheless, the risk of cross-reactivity is sufficient to warrant the contraindication of all
A Jarisch–Herxheimer reaction may occur after initial treatment of a spirochetal infection such as syphilis with a
Mechanism of action
[edit]Inhibition of cell wall synthesis
[edit]
Under normal circumstances, peptidoglycan precursors signal a reorganisation of the bacterial cell wall and, as a consequence, trigger the activation of autolytic cell wall hydrolases. Inhibition of cross-linkage by
Guanine oxidation
[edit]Another possibility that has been proposed to account for much of the cytotoxicity of beta lactams focuses on the oxidation of the guanine nucleotide in the bacterial nucleotide pool.[15] The incorporation of oxidized guanine nucleotide into DNA could cause cytotoxicity. Bacterial cytotoxicity could arise from incomplete repair of closely spaced 8-oxo-2'-deoxyguanosine lesions in the DNA resulting in double-strand breaks.[15]
Potency
[edit]Two structural features of
Modes of resistance
[edit]By definition, all
Enzymatic hydrolysis of the β -lactam ring
[edit]If the bacterium produces the enzyme


The production of a
Other
However, in all cases where infection with
In the context of medical pharmacology, penicillins, cephalosporins, and carbapenems, while all have the
Possession of altered penicillin-binding proteins
[edit]As a response to the use of
Nomenclature
[edit]
β -lactams fused to saturated five-membered rings:β -lactams containing thiazolidine rings are named penams.β -lactams containing pyrrolidine rings are named carbapenams.β -lactams fused to oxazolidine rings are named oxapenams or clavams.
β -lactams fused to unsaturated five-membered rings:β -lactams containing 2,3-dihydrothiazole rings are named penems.β -lactams containing 2,3-dihydro-1H-pyrrole rings are named carbapenems.
β -lactams fused to unsaturated six-membered rings:β -lactams containing 3,6-dihydro-2H-1,3-thiazine rings are named cephems.β -lactams containing 1,2,3,4-tetrahydropyridine rings are named carbacephems.β -lactams containing 3,6-dihydro-2H-1,3-oxazine rings are named oxacephems.
β -lactams not fused to any other ring are named monobactams.
By convention, the bicyclic
The numbering of monobactams follows that of the IUPAC; the nitrogen atom is position 1, the carbonyl carbon is 2, the
Biosynthesis
[edit]To date, two distinct methods of biosynthesizing the
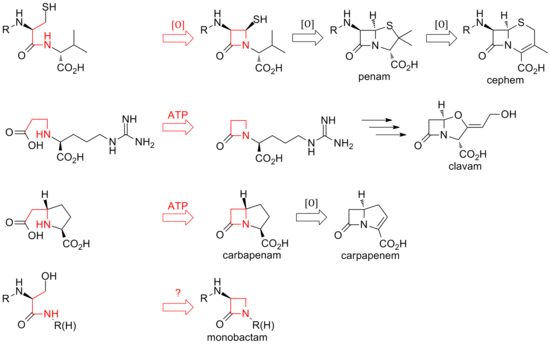
The biosynthesis of cephems branch off at isopenicillin N by an oxidative ring expansion to the cephem core. As with the penams, the variety of cephalosporins and cephamycins come from different transamidations, as is the case for the penicillins.[citation needed]
While the ring closure in penams and cephems is between positions 1 and 4 of the
The biosynthesis of the
See also
[edit]- List of
β -lactam antibiotics - ATC code J01C Beta-lactam antibacterials, penicillins
- ATC code J01D Other beta-lactam antibacterials
- Bacteria
- Cell wall
- Discovery and development of cephalosporins
- History of penicillin
- Nitrocefin
References
[edit]- ^ Holten KB, Onusko EM (August 2000). "Appropriate prescribing of oral beta-lactam antibiotics". American Family Physician. 62 (3): 611–20. PMID 10950216. Archived from the original on June 6, 2011. Retrieved November 8, 2008.
- ^ Yao, JDC, Moellering, RC Jr. (2007). "Antibacterial agents". In Murray, PR, et al. (eds.). Manual of Clinical Microbiology (9th ed.). Washington D.C.: ASM Press. Cited in Non-Penicillin Beta Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination (Report). U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). April 2013. Archived from the original on December 13, 2019. Retrieved May 27, 2019 – via US FDA website.
- ^ Elander RP (2003). "Industrial production of
β -lactam antibiotics". Applied Microbiology and Biotechnology. 61 (5–6): 385–392. doi:10.1007/s00253-003-1274-y. PMID 12679848. S2CID 43996071. - ^ Houbraken J, Frisvad JC, Samson RA (2011). "Fleming's penicillin producing strain is not Penicillium chrysogenum but P. rubens". IMA Fungus. 2 (1): 87–95. doi:10.5598/imafungus.2011.02.01.12. PMC 3317369. PMID 22679592.
- ^ Pathak A, Nowell RW, Wilson CG, Ryan MJ, Barraclough TG (September 2020). "Comparative genomics of Alexander Fleming's original Penicillium isolate (IMI 15378) reveals sequence divergence of penicillin synthesis genes". Scientific Reports. 10 (1): Article 15705. Bibcode:2020NatSR..1015705P. doi:10.1038/s41598-020-72584-5. PMC 7515868. PMID 32973216.
- ^ a b Pandey N, Cascella M (2020). "Beta lactam antibiotics". StatPearls. PMID 31424895. Archived from the original on December 15, 2020. Retrieved May 5, 2020.
- ^ Nau R, Sörgel F, Eiffert H (October 2010). "Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections". Clinical Microbiology Reviews. 23 (4): 858–83. doi:10.1128/CMR.00007-10. PMC 2952976. PMID 20930076.
- ^ a b c Rossi S (ed.) (2004). Australian Medicines Handbook 2004. Adelaide: Australian Medicines Handbook. ISBN 0-9578521-4-2.
- ^ Pichichero ME (April 2005). "A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients". Pediatrics. 115 (4): 1048–57. doi:10.1542/peds.2004-1276. PMID 15805383. S2CID 21246804.
- ^ Liccardi G, Caminati M, Senna G, Calzetta L, Rogliani P (October 2017). "Anaphylaxis and intimate behaviour". Current Opinion in Allergy and Clinical Immunology. 17 (5): 350–355. doi:10.1097/ACI.0000000000000386. ISSN 1473-6322. PMID 28742538. S2CID 13925217. Archived from the original on April 25, 2023. Retrieved February 27, 2021.
- ^ a b Miyachiro MM, Contreras-Martel C, Dessen A (2019). "Penicillin-Binding Proteins (PBPS) and Bacterial Cell Wall Elongation Complexes". Macromolecular Protein Complexes II: Structure and Function. Subcellular Biochemistry. Vol. 93. pp. 273–289. doi:10.1007/978-3-030-28151-9_8. ISBN 978-3-030-28150-2. PMID 31939154. S2CID 210814189.
- ^ Cushnie TP, O'Driscoll NH, Lamb AJ (2016). "Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action". Cellular and Molecular Life Sciences. 73 (23): 4471–4492. doi:10.1007/s00018-016-2302-2. hdl:10059/2129. PMC 11108400. PMID 27392605. S2CID 2065821. Archived from the original on October 7, 2017. Retrieved May 5, 2020.
- ^ Fisher JF, Meroueh SO, Mobashery S (2005). "Bacterial resistance to
β -lactam antibiotics: compelling opportunism, compelling opportunity". Chemical Reviews. 105 (2): 395–424. doi:10.1021/cr030102i. PMID 15700950. - ^ Kasten B, Reski R (January 1, 1997). "
β -Lactam antibiotics inhibit chloroplast division in a moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum)". Journal of Plant Physiology. 150 (1): 137–140. Bibcode:1997JPPhy.150..137K. doi:10.1016/S0176-1617(97)80193-9. - ^ a b Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC (April 20, 2012). "Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics". Science. 336 (6079): 315–319. Bibcode:2012Sci...336..315F. doi:10.1126/science.1219192. PMC 3357493. PMID 22517853.
- ^ a b Nangia A, Biradha K, Desiraju GR (1996). "Correlation of biological activity in
β -lactam antibiotics with Woodward and Cohen structural parameters—a Cambridge database study". Journal of the Chemical Society, Perkin Transactions 2 (5): 943–953. doi:10.1039/p29960000943. ISSN 1364-5471. - ^ Woodward RB (May 16, 1980). "Penems and related substances". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 289 (1036): 239–250. Bibcode:1980RSPTB.289..239W. doi:10.1098/rstb.1980.0042. ISSN 0962-8436. PMID 6109320.
- ^ Cohen NC (February 1, 1983). ".beta.-Lactam antibiotics: geometrical requirements for antibacterial activities". Journal of Medicinal Chemistry. 26 (2): 259–264. doi:10.1021/jm00356a027. ISSN 0022-2623. PMID 6827544.
- ^ Drawz SM, Bonomo RA (2010). "Three decades of
β -lactamase inhibitors". Clinical Microbiology Reviews. 23 (1): 160–201. doi:10.1128/CMR.00037-09. PMC 2806661. PMID 20065329. - ^ Gin A, Dilay L, Karlowsky JA, Walkty A, Rubinstein E, Zhanel GG (June 2007). "Piperacillin–tazobactam: a
β -lactam/β -lactamase inhibitor combination". Expert Review of Anti-infective Therapy. 5 (3): 365–383. doi:10.1586/14787210.5.3.365. ISSN 1478-7210. PMID 17547502. S2CID 68837323. - ^ Leonard DA, Bonomo RA, Powers RA (November 19, 2013). "Class D
β -Lactamases: a reappraisal after five decades". Accounts of Chemical Research. 46 (11): 2407–2415. doi:10.1021/ar300327a. ISSN 0001-4842. PMC 4018812. PMID 23902256. - ^ Macdougall C (2011). "Beyond susceptible and resistant Part I: treatment of infections due to Gram-negative organisms with inducible B-lactamases". Journal of Pediatric Pharmacology and Therapeutics. 16 (1): 23–30. doi:10.5863/1551-6776-16.1.23. PMC 3136230. PMID 22477821.
- ^ Kim D, Kim S, Kwon Y, Kim Y, Park H, Kwak K, Lee H, Lee JH, Jang KM, Kim D, Lee SH, Kang LW (March 2023). "Structural Insights for
β -Lactam Antibiotics". Biomol Ther (Seoul). 31 (2): 141–147. doi:10.4062/biomolther.2023.008. PMC 9970833. PMID 36788654. - ^ Ahmadvand P, Avillan JJ, Lewis JA, Call DR, Kang C (May 2022). "Characterization of Interactions between CTX-M-15 and Clavulanic Acid, Desfuroylceftiofur, Ceftiofur, Ampicillin, and Nitrocefin". Int J Mol Sci. 23 (9): 5229. doi:10.3390/ijms23095229. PMC 9100253. PMID 35563620.
- ^ "Archived copy" (PDF). Archived (PDF) from the original on February 16, 2022. Retrieved February 22, 2024.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Christenson JC, Korgenski EK (2008). "Laboratory Diagnosis of Infection Due to Bacteria, Fungi, Parasites, and Rickettsiae". Principles and Practice of Pediatric Infectious Disease. W.B. Saunders. pp. 1341–1352. doi:10.1016/B978-0-7020-3468-8.50292-3. ISBN 978-0-7020-3468-8.
- ^ a b "Archived copy". Archived from the original on February 22, 2024. Retrieved February 22, 2024.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ a b Palzkill T (January 2013). "Metallo-
β -lactamase structure and function". Ann N Y Acad Sci. 1277 (1): 91–104. Bibcode:2013NYASA1277...91P. doi:10.1111/j.1749-6632.2012.06796.x. PMC 3970115. PMID 23163348. - ^ a b Fawaz S, Barton S, Whitney L, Swinden J, Nabhani-Gebara S (2019). "Stability of Meropenem After Reconstitution for Administration by Prolonged Infusion". Hospital Pharmacy. 54 (3): 190–196. doi:10.1177/0018578718779009. PMC 6535930. PMID 31205331. Archived from the original on February 27, 2024. Retrieved February 22, 2024.
- ^ "Ceftriaxone: Package Insert". Archived from the original on April 2, 2023.
- ^ https://web.archive.org/web/20240222172948/https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/0550585s063lbl.pdf [bare URL PDF]
- ^ Ubukata K, Nonoguchi R, Matsuhashi M, Konno M (1989). "Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein". Journal of Bacteriology. 171 (5): 2882–5. doi:10.1128/jb.171.5.2882-2885.1989. PMC 209980. PMID 2708325.
- ^ Dalhoff A, Janjic N, Echols R (2006). "Redefining penems". Biochemical Pharmacology. 71 (7): 1085–1095. doi:10.1016/j.bcp.2005.12.003. PMID 16413506.
- ^ Lundberg M, Siegbahn PE, Morokuma K (2008). "The mechanism for isopenicillin N synthase from density-functional modeling highlights the similarities with other enzymes in the 2-His-1-carboxylate family". Biochemistry. 47 (3): 1031–1042. doi:10.1021/bi701577q. PMID 18163649. Archived from the original on September 22, 2017. Retrieved October 28, 2017.
- ^ Bachmann BO, Li R, Townsend CA (1998). "
β -lactam synthetase: a new biosynthetic enzyme". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9082–9086. Bibcode:1998PNAS...95.9082B. doi:10.1073/pnas.95.16.9082. PMC 21295. PMID 9689037. - ^ Townsend CA, Brown AM, Nguyen LT (1983). "Nocardicin A: stereochemical and biomimetic studies of monocyclic
β -lactam formation". Journal of the American Chemical Society. 105 (4): 919–927. doi:10.1021/ja00342a047.


