Cleanroom






A cleanroom or clean room is an engineered space that maintains a very low concentration of airborne particulates. It is well isolated, well controlled from contamination, and actively cleansed. Such rooms are commonly needed for scientific research and in industrial production for all nanoscale processes, such as semiconductor manufacturing. A cleanroom is designed to keep everything from dust to airborne organisms or vaporised particles away from it, and so from whatever material is being handled inside it.
A cleanroom can also prevent the escape of materials. This is often the primary aim in hazardous biology, nuclear work, pharmaceutics and virology.
Cleanrooms typically come with a cleanliness level quantified by the number of particles per cubic meter at a predetermined molecule measure. The ambient outdoor air in a typical urban area contains 35,000,000 particles for each cubic meter in the size range 0.5
History
[edit]The modern cleanroom was invented by American physicist Willis Whitfield.[1] As an employee of the Sandia National Laboratories, Whitfield created the initial plans for the cleanroom in 1960.[1] Prior to Whitfield's invention, earlier cleanrooms often had problems with particles and unpredictable airflows. Whitfield designed his cleanroom with a constant, highly filtered airflow to flush out impurities.[1] Within a few years of its invention in the 1960s, Whitfield's modern cleanroom had generated more than US$50 billion in sales worldwide (approximately $483 billion today).[2][3]
By mid-1963, more than 200 U.S. industrial plants had such specially constructed facilities—then using the terminology “White Rooms,” “Clean Rooms,” or “Dust-Free Rooms”—including the Radio Corporation of America, McDonnell Aircraft, Hughes Aircraft, Sperry Rand, Sylvania Electric, Western Electric, Boeing, and North American Aviation.[4] RCA began such a conversion of part of its Cambridge, Ohio facilities in February 1961. Totalling 70,000 square feet, it was used to prepare control equipment for the Minuteman ICBM missiles.[5]
The majority of the integrated circuit manufacturing facilities in Silicon Valley were made by three companies: MicroAire, PureAire, and Key Plastics. These competitors made laminar flow units, glove boxes, cleanrooms and air showers, along with the chemical tanks and benches used in the "wet process" building of integrated circuits. These three companies were the pioneers of the use of Teflon for airguns, chemical pumps, scrubbers, water guns, and other devices needed for the production of integrated circuits. William (Bill) C. McElroy Jr. worked as an engineering manager, drafting room supervisor, QA/QC, and designer for all three companies, and his designs added 45 original patents to the technology of the time. McElroy also wrote a four-page article for MicroContamination Journal, wet processing training manuals, and equipment manuals for wet processing and cleanrooms.[6][citation needed]
Overview
[edit]A cleanroom is a necessity in the manufacturing of semiconductors and rechargeable batteries, the life sciences, and any other field that is highly sensitive to environmental contamination.
Cleanrooms can range from the very small to the very large. On the one hand, a single-user laboratory can be built to cleanroom standards within several square meters, and on the other, entire manufacturing facilities can be contained within a cleanroom with factory floors covering thousands of square meters. Between the large and the small, there are also modular cleanrooms.[7] They have been argued to lower costs of scaling the technology, and to be less susceptible to catastrophic failure.
With such a wide area of application, not every cleanroom is the same. For example, the rooms utilized in semiconductor manufacturing need not be sterile (i.e., free of uncontrolled microbes),[8] while the ones used in biotechnology usually must be. Vice versa, operating rooms need not be absolutely pure of nanoscale inorganic salts, such as rust, while nanotechnology absolutely requires it. What then is common to all cleanrooms is strict control of airborne particulates, possibly with secondary decontamination of air, surfaces, workers entering the room, implements, chemicals, and machinery.
Sometimes particulates exiting the compartment are also of concern, such as in research into dangerous viruses, or where radioactive materials are being handled.
Basic construction
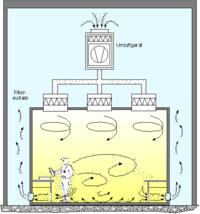
[edit]First, outside air entering a cleanroom is filtered and cooled by several outdoor air handlers using progressively finer filters to exclude dust.
Within, air is constantly recirculated through fan units containing high-efficiency particulate absorbing filters (HEPA), and/or ultra-low particulate air (ULPA) filters to remove internally generated contaminants. Special lighting fixtures, walls, equipment and other materials are used to minimize the generation of airborne particles. Plastic sheets can be used to restrict air turbulence if the cleanroom design is of the laminar airflow type.[9][10][11]
Air temperature and humidity levels inside a cleanroom are tightly controlled, because they affect the efficiency and means of air filtration. If a particular room requires low enough humidity to make static electricity a concern, it too will be controlled by, e.g., introducing controlled amounts of charged ions into the air using a corona discharge. Static discharge is of particular concern in the electronics industry, where it can instantly destroy components and circuitry.
Equipment inside any cleanroom is designed to generate minimal air contamination. The selection of material for the construction of a cleanroom should not generate any particulates; hence, monolithic epoxy or polyurethane floor coating is preferred. Buffed stainless steel or powder-coated mild steel sandwich partition panels and ceiling panel are used instead of iron alloys prone to rusting and then flaking. Corners like the wall to wall, wall to floor, wall to ceiling are avoided by providing coved surface, and all joints need to be sealed with epoxy sealant to avoid any deposition or generation of particles at the joints, by vibration and friction. Many cleanrooms have a "tunnel" design in which there are spaces called "service chases" that serve as air plenums carrying the air from the bottom of the room to the top so that it can be recirculated and filtered at the top of the cleanroom.[12]
Airflow principles
[edit]Cleanrooms maintain particulate-free air through the use of either HEPA or ULPA filters employing laminar or turbulent airflow principles. Laminar, or unidirectional, airflow systems direct filtered air downward or in horizontal direction in a constant stream towards filters located on walls near the cleanroom floor or through raised perforated floor panels to be recirculated. Laminar airflow systems are typically employed across 80% of a cleanroom ceiling to maintain constant air processing. Stainless steel or other non shedding materials are used to construct laminar airflow filters and hoods to prevent excess particles entering the air. Turbulent, or non-unidirectional, airflow uses both laminar airflow hoods and nonspecific velocity filters to keep air in a cleanroom in constant motion, although not all in the same direction. The rough air seeks to trap particles that may be in the air and drive them towards the floor, where they enter filters and leave the cleanroom environment. US FDA and EU have laid down stringent guidelines and limits to ensure freedom from microbial contamination in pharmaceutical products.[13] Plenums between air handlers and fan filter units, along with sticky mats, may also be used.
In addition to air filters, cleanrooms can also use ultraviolet light to disinfect the air.[14] UV devices can be fitted into ceiling light fixtures and irradiate air, killing potentially infectious particulates, including 99.99 percent of airborne microbial and fungal contaminants.[15] UV light has previously been used to clean surface contaminants in sterile environments such as hospital operating rooms. Their use in other cleanrooms may increase as equipment becomes more affordable. Potential advantages of UV-based decontamination includes a reduced reliance on chemical disinfectants and the extension of HVAC filter life.
Cleanrooms of different kinds
[edit]Some cleanrooms are kept at a positive pressure so if any leaks occur, air leaks out of the chamber instead of unfiltered air coming in. This is most typically the case in semiconductor manufacturing, where even minute amounts of particulates leaking in could contaminate the whole process, while anything leaking out would not be harmful to the surrounding community[citation needed]. The opposite is done, e.g., in the case of high-level bio-laboratories that handle dangerous bacteria or viruses; those are always held at negative pressure, with the exhaust being passed through high-efficiency filters, and further sterilizing procedures. Both are still cleanrooms because the particulate level inside is maintained within very low limits.
Some cleanroom HVAC systems control the humidity to such low levels that extra equipment like air ionizers are required to prevent electrostatic discharge problems. This is a particular concern within the semiconductor business, because static discharge can easily damage modern circuit designs. On the other hand, active ions in the air can harm exposed components as well. Because of this, most workers in high electronics and semiconductor facilities have to wear conductive boots while working. Low-level cleanrooms may only require special shoes, with completely smooth soles that do not track in dust or dirt. However, for safety reasons, shoe soles must not create slipping hazards. Access to a cleanroom is usually restricted to those wearing a cleanroom suit, including the necessary machinery.
In cleanrooms in which the standards of air contamination are less rigorous, the entrance to the cleanroom may not have an air shower. An anteroom (known as a "gray room") is used to put on cleanroom clothing. This practice is common in many nuclear power plants, which operate as low-grade inverse pressure cleanrooms, as a whole.
Recirculating vs. one pass cleanrooms
Recirculating cleanrooms return air to the negative pressure plenum via low wall air returns. The air then is pulled by HEPA fan filter units back into the cleanroom. The air is constantly recirculating and by continuously passing through HEPA filtration removing particles from the air each time. Another advantage of this design is that air conditioning can be incorporated.
One pass cleanrooms draw air from outside and pass it through HEPA fan filter units into the cleanroom. The air then leaves through exhaust grills. The advantage of this approach is the lower cost. The disadvantages are comparatively shorter HEPA fan filter life, worse particle counts than a recirculating cleanroom, and that it cannot accommodate air conditioning.
Operating procedure
[edit]In order to minimize the carrying of particulate by a person moving into the cleanroom, staff enter and leave through airlocks (sometimes including an air shower stage) and wear protective clothing such as hoods, face masks, gloves, boots, and coveralls.
Common materials such as paper, pencils, and fabrics made from natural fibers are often excluded because they shed particulates in use.
Particle levels are usually tested using a particle counter and microorganisms detected and counted through environmental monitoring methods[clarify].[16][17] Polymer tools used in cleanrooms must be carefully determined to be chemically compatible with cleanroom processing fluids [18] as well as ensured to generate a low level of particle generation.[19]
When cleaning, only special mops and buckets are used. Cleaning chemicals used tend to involve sticky elements to trap dust, and may need a second step with light molecular weight solvents to clear. Cleanroom furniture is designed to produce a minimum of particles and is easy to clean.
A cleanroom is as much a process and a meticulous culture to maintain, as it is a space as such.
Personnel contamination of cleanrooms
[edit]The greatest threat to cleanroom contamination comes from the users themselves.[20] In the healthcare and pharmaceutical sectors, control of microorganisms is important, especially microorganisms likely to be deposited into the air stream from skin shedding. Studying cleanroom microflora is of importance for microbiologists and quality control personnel to assess changes in trends. Shifts in the types of microflora may indicate deviations from the "norm" such as resistant strains or problems with cleaning practices.
In assessing cleanroom microorganisms, the typical flora are primarily those associated with human skin (Gram-positive cocci), although microorganisms from other sources such as the environment (Gram-positive rods) and water (Gram-negative rods) are also detected, although in lower number. Common bacterial genera include Micrococcus, Staphylococcus, Corynebacterium, and Bacillus, and fungal genera include Aspergillus and Penicillium.[17]
Cleanroom classification and standardization
[edit]
Cleanrooms are classified according to the number and size of particles permitted per volume of air. Large numbers like "class 100" or "class 1000" refer to FED-STD-209E, and denote the number of particles of size 0.5
A discrete, light-scattering airborne particle counter is used to determine the concentration of airborne particles, equal to and larger than the specified sizes, at designated sampling locations.
Small numbers refer to ISO 14644-1 standards, which specify the decimal logarithm of the number of particles 0.1
Both FS 209E and ISO 14644-1 assume log-log relationships between particle size and particle concentration. For that reason, zero particle concentration does not exist. Some classes do not require testing some particle sizes, because the concentration is too low or too high to be practical to test for, but such blanks should not be read as zero.
Because 1 m3 is about 35 ft3, the two standards are mostly equivalent when measuring 0.5
ISO 14644-1 and ISO 14698
[edit]ISO 14644-1 and ISO 14698 are non-governmental standards developed by the International Organization for Standardization (ISO).[23] The former applies to cleanrooms in general (see table below), the latter to cleanrooms where biocontamination may be an issue. Since the strictest standards have been achieved only for space applications, it is sometimes difficult to know whether they were achieved in vacuum or standard conditions.
ISO 14644-1 defines the maximum concentration of particles per class and per particle size with the following formula[24]
Where is the maximum concentration of particles in a volume of 1m of airborne particles that are equal to, or larger, than the considered particle size which is rounded to the nearest whole number, using no more than three significant figures, is the ISO class number, is the size of the particle in m and 0.1 is a constant expressed in m. The result for standard particle sizes is expressed in the following table.
| Class | Maximum particles/m3 a | FED STD 209E equivalent | |||||
|---|---|---|---|---|---|---|---|
| ≥0.1 |
≥0.2 |
≥0.3 |
≥0.5 |
≥1 |
≥5 | ||
| ISO 1 | 10b | d | d | d | d | e | |
| ISO 2 | 100 | 24b | 10b | d | d | e | |
| ISO 3 | 1,000 | 237 | 102 | 35b | d | e | Class 1 |
| ISO 4 | 10,000 | 2,370 | 1,020 | 352 | 83b | e | Class 10 |
| ISO 5 | 100,000 | 23,700 | 10,200 | 3,520 | 832 | d,e,f | Class 100 |
| ISO 6 | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 | Class 1,000 |
| ISO 7 | c | c | c | 352,000 | 83,200 | 2,930 | Class 10,000 |
| ISO 8 | c | c | c | 3,520,000 | 832,000 | 29,300 | Class 100,000 |
| ISO 9 | c | c | c | 35,200,000 | 8,320,000 | 293,000 | Room air |
a All concentrations in the table are cumulative, e.g. for ISO Class 5, the 10 200 particles shown at 0,3
b These concentrations will lead to large air sample volumes for classification. Sequential sampling procedure may be applied; see Annex D. | |||||||
US FED STD 209E
[edit]US FED-STD-209E was a United States federal standard. It was officially cancelled by the General Services Administration on November 29, 2001,[25][26] but is still widely used.[27]
| Class | Maximum particles/ft3 | ISO equivalent | ||||
|---|---|---|---|---|---|---|
| ≥0.1 |
≥0.2 |
≥0.3 |
≥0.5 |
≥5 | ||
| 1 | 35 | 7.5 | 3 | 1 | 0.007 | ISO 3 |
| 10 | 350 | 75 | 30 | 10 | 0.07 | ISO 4 |
| 100 | 3,500 | 750 | 300 | 100 | 0.7 | ISO 5 |
| 1,000 | 35,000 | 7,500 | 3000 | 1,000 | 7 | ISO 6 |
| 10,000 | 350,000 | 75,000 | 30,000 | 10,000 | 70 | ISO 7 |
| 100,000 | 3.5×106 | 750,000 | 300,000 | 100,000 | 830 | ISO 8 |
Current regulating bodies include ISO, USP 800, US FED STD 209E (previous standard, still used).
- Drug Quality and Security Act (DQSA) created in Nov. 2013 in response to drug compounding deaths and serious adverse events.
- The Federal Food, Drug, and Cosmetic Act (FD&C Act) created specific guidelines and policies for human compounding.
- 503A addresses compounding by state or federally licensed facility by licensed personnel (pharmacist/ physicians)
- 503B pertaining to outsourcing facilities need direct supervision from a licensed pharmacist and do not need to be a licensed pharmacy. Facility is licensed through the Food and Drug Administration (FDA) [28]
EU GMP classification
[edit]EU GMP guidelines are more stringent than others, requiring cleanrooms to meet particle counts at operation (during manufacturing process) and at rest (when manufacturing process is not carried out, but room AHU is on).
| Class | Maximum particles/m3[29] | |||
|---|---|---|---|---|
| At Rest | In Operation | |||
| 0.5 |
5.0 |
0.5 |
5.0 | |
| Grade A | 3,520 | 20 | 3,520 | 20 |
| Grade B | 3,520 | 29 | 352,000 | 2,900 |
| Grade C | 352,000 | 2,900 | 3,520,000 | 29,000 |
| Grade D | 3,520,000 | 29,000 | Not defined | Not defined |
BS 5295
[edit]BS 5295 is a British Standard.
| Class | Maximum particles/m3 | |||||
|---|---|---|---|---|---|---|
| ≥0.5 |
≥1 |
≥5 |
≥10 |
≥25 | ||
| Class 1 | 3,000 | 0 | 0 | 0 | ||
| Class 2 | 300,000 | 2,000 | 30 | |||
| Class 3 | 1,000,000 | 20,000 | 4,000 | 300 | ||
| Class 4 | 200,000 | 40,000 | 4,000 | |||
BS 5295 Class 1 also requires that the greatest particle present in any sample can not exceed 5
USP <800> standards
[edit]USP 800 is a United States standard developed by the United States Pharmacopeial Convention (USP) with an effective date of December 1, 2019.[32]
Ramifications and further applications
[edit]In hospitals, theatres are similar to cleanrooms for surgical patients' operations with incisions to prevent any infections for the patient.
In another case, severely immunocompromised patients sometimes have to be held in prolonged isolation from their surroundings, for fear of infection. At the extreme, this necessitates a cleanroom environment. The same is the case for patients carrying airborne infectious diseases, only they are handled at negative, not positive pressure.
In exobiology when we seek out contact with other planets, there is a biological hazard both ways: we must not contaminate any sample return missions from other stellar bodies with terrestrial microbes, and we must not contaminate possible other ecosystems existing in other planets. Thus, even by international law, any probes we send to outer space must be sterile, and so to be handled in cleanroom conditions.[citation needed]
Since larger cleanrooms are very sensitive controlled environments upon which multibillion-dollar industries depend, sometimes they are even fitted with numerous seismic base isolation systems to prevent costly equipment malfunction.[33]
See also
[edit]- Air ionizer
- Air conditioning
- Air quality index
- Contamination control
- Data recovery lab
- Indoor air quality
- Microfiltration
- Pressure room (disambiguation)
- Particle counter
- Pneumatic filter
- Secure environment
- Semiconductor device fabrication
References
[edit]- ^ a b c Yardley, William (2012-12-04). "Willis Whitfield, Clean Room Inventor, Dies at 92". The New York Times. Retrieved 2013-06-22.[permanent dead link]
- ^ "Sandia physicist, cleanroom inventor dies at 92". KWES. Associated Press. 2012-11-26. Retrieved 2012-12-03.
- ^ "Willis Whitfield - Father of the Cleanroom" (PDF). Cleanroom online. September 2015. Archived from the original (PDF) on 2021-01-27. Retrieved 2016-05-18.
- ^ Koslow, Jules. “Industry’s Pursuit of Cleanliness.” Electronic Age 22:3 (Summer 1963), 22-25.
- ^ Koslow, Jules. “Industry’s Pursuit of Cleanliness.” Electronic Age 22:3 (Summer 1963), 22-25.
- ^ William (Bill) C. McElroy Jr., MicroAire Engineering Manager and acting VP; Kay Plastics Engineering Manager; PureAire Drafting Room Manager
- ^ "What is a Cleanroom? | Mecart". MECART Cleanrooms. August 16, 2016.
- ^ In NASA's Sterile Areas, Plenty of Robust Bacteria New York Times, 9. October 2007
- ^ "Kenall Cleanroom and Containment Lighting". kenall.com.
- ^ "Fitting lights in cleanrooms". www.cleanroomtechnology.com.
- ^ "Cleanroom LED". Philips.
- ^ "Lecture 30: Cleanroom design and contamination control" (PDF). najah.edu. Retrieved 1 March 2024.
- ^ "Cleanroom Air Flow Principles". www.thomasnet.com.
- ^ Guimera, Don; Trzil, Jean; Joyner, Joy; Hysmith, Nicholas D. (2018-02-01). "Effectiveness of a shielded ultraviolet C air disinfection system in an inpatient pharmacy of a tertiary care children's hospital". American Journal of Infection Control. 46 (2): 223–225. doi:10.1016/j.ajic.2017.07.026. ISSN 0196-6553. PMID 28865936.
- ^ outsourcing-pharma.com. "UV helps maintain cleanroom air quality". outsourcing-pharma.com. Retrieved 2020-04-15.
- ^ Sandle, T (November 2012). "Application of quality risk management to set viable environmental monitoring frequencies in biotechnology processing and support areas". PDA J Pharm Sci Technol. 66 (6): 560–79. doi:10.5731/pdajpst.2012.00891. PMID 23183652. S2CID 7970.
- ^ a b Sandle, T (November 2011). "A review of cleanroom microflora: types, trends, and patterns". PDA J Pharm Sci Technol. 65 (4): 392–403. doi:10.5731/pdajpst.2011.00765. PMID 22293526. S2CID 25970142.
- ^ Heikkinen, Ismo T.S.; Kauppinen, Christoffer; Liu, Zhengjun; Asikainen, Sanja M.; Spoljaric, Steven; Seppälä, Jukka V.; Savin, Hele (October 2018). "Chemical compatibility of fused filament fabrication-based 3-D printed components with solutions commonly used in semiconductor wet processing" (PDF). Additive Manufacturing. 23: 99–107. doi:10.1016/j.addma.2018.07.015. ISSN 2214-8604. S2CID 139867946.
- ^ Pasanen, T.P.; von Gastrow, G.; Heikkinen, I.T.S.; Vähänissi, V.; Savin, H.; Pearce, J.M. (January 2019). "Compatibility of 3-D printed devices in cleanroom environments for semiconductor processing". Materials Science in Semiconductor Processing. 89: 59–67. doi:10.1016/j.mssp.2018.08.027. ISSN 1369-8001. S2CID 105579272.
- ^ "Cleanroom and Controlled Environment Attire - ANSI Blog". The ANSI Blog. 2015-07-15. Retrieved 2018-11-24.
- ^ "Space Station Processing Facility (SSPF)". science.ksc.nasa.gov.
- ^ "Cleanroom Classification / Particle Count / FS209E / ISO TC209 /". Archived from the original on 2008-02-14. Retrieved 2008-03-05.
- ^ "ISO 14644-1:2015 - Cleanrooms and associated controlled environments -- Part 1: Classification of air cleanliness by particle concentration". ISO. 21 January 2016. Retrieved 2016-09-12.
- ^ W. Whyte (17 October 2001). Cleanroom Technology: Fundamentals of Design, Testing and Operation. John Wiley & Sons. ISBN 978-0-471-86842-2.
- ^ "Federal Standard 209E Cancellation". www.iest.org.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 2008-05-28. Retrieved 2008-04-17.
{{cite web}}: CS1 maint: archived copy as title (link), page 148 - ^ "NUFAB SAFETY & PROTOCOL" (PDF). Retrieved 24 February 2016.
- ^ Research, Center for Drug Evaluation and (2019-02-09). "FD&C Act Provisions that Apply to Human Drug Compounding". FDA.
- ^ "Understanding Cleanroom Classifications". Archived from the original on 2016-06-01. Retrieved 2015-08-21.
- ^ Market Venture Philippines Inc. web site (2006-04-19). "What is a Clean Room?". Archived from the original on 2012-08-28. Retrieved 2007-06-02.
- ^ "BS 5295-0:1989 - Environmental cleanliness in enclosed spaces. General introduction, terms and definitions for clean rooms and clean air devices". 2016. Retrieved 2016-04-18.
- ^ "USP 800 | USP". www.usp.org. Retrieved 2020-04-13.
- ^ "Semiconductor Technology at TSMC, 2011". 26 March 2011. Archived from the original on 2021-12-12 – via YouTube.