亚碲酸 钠
| 亚碲 | |
|---|---|

| |
| |
| 别名 | Sodium Tellurite IV, Tellurous acid disodium salt |
| 识别 | |
| CAS |
10102-20-2 |
| PubChem | 24935 |
| ChemSpider | 23309 |
| SMILES |
|
| InChI |
|
| InChIKey | VOADVZVYWFSHSM-NUQVWONBAS |
| EINECS | 233-268-4 |
| RTECS | WY2450000 |
| Na2TeO3 | |
| 221.57774 g·mol⁻¹ | |
| 6.245 g/cm3 | |
| 熔点 | 710 °C(983 K) |
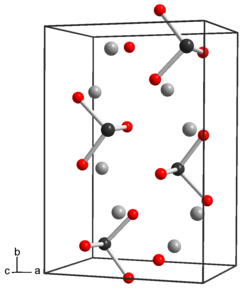
| 结构 | |
| 危险 | |
| 警示术语 | R:R23 R24 R25 |
| S:S22 S36 S37 S39 S45 | |
LD50(
|
83 mg/kg (rat, oral) |
亚碲
制 备
[编辑]碲的
- Ag2Te + Na2CO3 + O2 →2Ag + Na2TeO3 + CO2 (400-500°C)
这是
纯化
[编辑]- 阳极:4OH - →2H2O + O2 + 4e -
- 阴极:TeO3 2- + 3H2O + 4e - →Te + 6OH -
结构和 性 质
[编辑]碲
碲酸盐相关的反 应
[编辑]- H2TeO3 → H+ + HTeO3− pK2.48
碲酸
- HTeO3− →H+ +TeO32− pK7.7
亚碲
- TeO2 + 2OH− → TeO32− + H2O
这是二氧化碲与碱反应生成亚碲酸盐的反应。
应用
[编辑]亚碲
参考 文献
[编辑]- ^ 1.0 1.1 Wiberg, Egon; Holleman, Arnold Frederick. Nils Wiberg , 编. Inorganic chemistry. translated by Mary Eagleson. Academic Press. 2001: 588. ISBN 0-12-352651-5.
- ^ Masse, R.; Guitel, J.C.; Tordjman, I. Preparation chimique et structure cristalline des tellurites de sodium et d'argent: Na2TeO3, Ag2TeO3. Materials Research Bulletin. 1980, 15 (4): 431–436. ISSN 0025-5408. doi:10.1016/0025-5408(80)90048-3.
- ^ Etude cristallographique du tellurite de sodium à cinq molécules d'eau, Na2TeIVO3·5H2O. Acta Crystallogr. B. 1979, 35: 1337–1340. doi:10.1107/S0567740879006403.
- ^ Borsetti, Francesca; Toninello, Antonio; Zannoni, Davide (2003). "Tellurite uptake by cells of the facultative phototroph Rhodobacter capsulatus is a pH-dependent process." Federation of European Biochemical Societies. Volume 554, Issue 3, 20 November 2003, pp. 315–318. Elsevier B.V. doi:10.1016/S0014-5793(03)01180-3
- Cameo Chemicals. Sodium Tellurite. Retrieved March 8, 2009. Website: http://cameochemical.noaa.gov/chemical/5185[
永久 失效 連結 ]. - Knockaert, Guy (2002), "Tellurium and Tellurium Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a26_177.pub2
