Fluoxymesterone: Difference between revisions
No edit summary |
|||
| Line 60: | Line 60: | ||
}} |
}} |
||
<!-- Definition and medical uses --> |
<!-- Definition and medical uses --> |
||
'''Fluoxymesterone''', sold under the brand names '''Halotestin''' and '''Ultandren''' among others, is an [[androgen]] and [[anabolic steroid]] (AAS) which is used in the treatment of |
'''Fluoxymesterone''', sold under the brand names '''Halotestin''' and '''Ultandren''' among others, is an [[androgen]] and [[anabolic steroid]] (AAS) which is used in the treatment of [[hypogonadism|low testosterone levels]] in men, [[delayed puberty]] in boys, [[breast cancer]] in women, and [[anemia]].<ref name="Llewellyn2011">{{cite book|author=William Llewellyn|title=Anabolics|url=https://books.google.com/books?id=afKLA-6wW0oC&pg=PT500|year=2011|publisher=Molecular Nutrition Llc|isbn=978-0-9828280-1-4|pages=500–508}}</ref> It is taken [[oral administration|by mouth]].<ref name="Llewellyn2011" /> |
||
<!-- Side effects and mechanism of action --> |
<!-- Side effects and mechanism of action --> |
||
Revision as of 03:37, 18 December 2017
 | |
| Clinical data | |
|---|---|
| Trade names | Halotestin, Ora-Testryl, Ultandren, others |
| Other names | Androfluorene; NSC-12165; 9 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682690 |
| Pregnancy category |
|
| Routes of administration | By mouth[1] |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 80%[2] |
| Metabolism | Liver (6 |
| Metabolites | • 5 • 11-Oxofluoxymesterone[3] |
| Elimination half-life | 9.2 hours[4][5] |
| Excretion | Urine (<5% unchanged)[2][3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.875 |
| Chemical and physical data | |
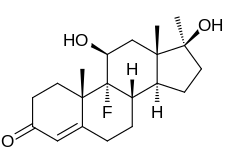
| Formula | C20H29FO3 |
| Molar mass | 336.441 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is an androgen and anabolic steroid (AAS) which is used in the treatment of low testosterone levels in men, delayed puberty in boys, breast cancer in women, and anemia.[1] It is taken by mouth.[1]
Side effects of fluoxymesterone include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[1] It can also cause liver damage and cardiovascular side effects like high blood pressure.[1][6][7] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1][8] It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization.[1]
Fluoxymesterone was first described in 1956 and was introduced for medical use in 1957.[1][9] In addition to its medical use, fluoxymesterone is used to improve physique and performance.[1] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[1]
Medical uses
Fluoxymesterone is or has been used in the treatment of hypogonadism, delayed puberty, and anemia in males and the treatment of breast cancer in women.[1][10] It is specifically approved in one or more countries for the treatment of hypogonadism in men, delayed puberty in boys, and breast cancer in women.[11] Current prescribing guidelines in the United States list only the treatment of androgen deficiency in males and breast cancer in females as indications.[1]
Side effects
Side effects that have been associated with fluoxymesterone include acne, edema, seborrhea/seborrheic dermatitis, alopecia, hirsutism, voice deepening, virilization in general, flushing, gynecomastia, breast pain, menstrual disturbances, hypogonadism, testicular atrophy, clitoral enlargement, penile enlargement, priapism, increased aggressiveness, prostate enlargement, cardiovascular toxicity, and hepatotoxicity, among others.[1][12]
Pharmacology
Pharmacodynamics
As an AAS, fluoxymesterone is an agonist of the androgen receptor (AR), similarly to androgens like testosterone and DHT.[1][13] It is a substrate for 5
Fluoxymesterone has been reported to be non-aromatizable due to steric hindrance by its C11
Because of the presence of its 17
11β -HSD inhibition
Fluoxymesterone has been found to act as a potent inhibitor of 11
Glucocorticoid activity
Unlike other AAS, fluoxymesterone has structural features in common with corticosteroids, including its C9
Pharmacokinetics
Fluoxymesterone has approximately 80% oral bioavailability, unlike testosterone, as the C17
Chemistry
Fluoxymesterone is an androstane steroid and a 17
Synthesis
Step one: The first step in the synthesis of fluoxymesterone is the microbiological oxidation of commercially available androstenedione (1.11) by Actinomyces; this introduces a hydroxyl group to the 11

Step two: The 11
Detection in body fluids
Detection of halotestin and other such illegal anabolic steroids in sports is achieved by GS-MS identification of urinary excreted anabolic steroids and their metabolites. In a test for halotestin, a dry residue obtained from a urine sample is dissolved in dimethylformamide and a sulfur trioxide-pyridine complex and is heated with 1% potassium carbonate solution. Halotestin and many of its metabolites contain two polar hydroxyl groups, leading to intermolecular hydrogen bonding that increases their boiling point and reduces volatility. In order to attain a gaseous sample for GC-MS, the products of hydrolysis are extracted, dissolved in methanol and derivatised to form volatile trimethylsilyl (TMS) esters by adding N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA) and trimethylsilylimidazole (TMSImi).[23]
History
Fluoxymesterone was first described in 1956 and was introduced for medical use in the United States in 1957.[1][9] Over time the use of fluoxymesterone has become increasingly controversial and limited.[1]
Society and culture
Generic names
Fluoxymesterone is the generic name of the drug and its INN, USP, BAN, DCIT, and JAN, while fluoxymestérone is its DCF.[21][22][24][25]
Brand names
Brand names of fluoxymesterone include Android-F, Androxy, Halotestin, Ora-Testryl, and Ultandren among others.[21][22][24][25]
Availability
United States
Fluoxymesterone is one of the few AAS that remains available for medical use in the United States.[26] The others (as of November 2017) are testosterone, testosterone cypionate, testosterone enanthate, testosterone undecanoate, methyltestosterone, nandrolone decanoate, oxandrolone, and oxymetholone.[26]
Other countries
Availability of fluoxymesterone aside from the United States remains scarce, but it is marketed in some other countries, such as Mexico, Moldova, and Taiwan.[1][25]
Legal status
Fluoxymesterone, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[27]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 500–508. ISBN 978-0-9828280-1-4.
- ^ a b c d Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1360–. ISBN 978-1-60913-345-0.
- ^ a b c d e f g h Kammerer RC, Merdink JL, Jagels M, Catlin DH, Hui KK (1990). "Testing for fluoxymesterone (Halotestin) administration to man: identification of urinary metabolites by gas chromatography-mass spectrometry". J. Steroid Biochem. 36 (6): 659–66. PMID 2214783.
- ^ a b Seth Roberts (2009). Anabolic Pharmacology.
- ^ Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1279–. ISBN 978-0-7817-6879-5.
- ^ a b c d e f Fürstenberger C, Vuorinen A, Da Cunha T, Kratschmar DV, Saugy M, Schuster D, Odermatt A (2012). "The anabolic androgenic steroid fluoxymesterone inhibits 11
β -hydroxysteroid dehydrogenase 2-dependent glucocorticoid inactivation". Toxicol. Sci. 126 (2): 353–61. doi:10.1093/toxsci/kfs022. PMID 22273746. - ^ a b c d Joseph JF, Parr MK (2015). "Synthetic androgens as designer supplements". Curr Neuropharmacol. 13 (1): 89–100. doi:10.2174/1570159X13666141210224756. PMC 4462045. PMID 26074745.
- ^ Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ a b William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1676–. ISBN 978-0-8155-1856-3.
- ^ Susan M. Ford; Sally S. Roach (7 October 2013). Roach's Introductory Clinical Pharmacology. Lippincott Williams & Wilkins. pp. 502–. ISBN 978-1-4698-3214-2.
- ^ http://adisinsight.springer.com/drugs/800012288
- ^ Jerome Z. Litt; Neil Shear (17 December 2014). Litt's Drug Eruptions and Reactions Manual, 19th Edition. CRC Press. pp. 177–. ISBN 978-1-84214-599-9.
- ^ a b c d e Kicman, A T (2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR (2008). "Dimethandrolone (7alpha,11beta-dimethyl-19-nortestosterone) and 11beta-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase". J. Steroid Biochem. Mol. Biol. 110 (3–5): 214–22. doi:10.1016/j.jsbmb.2007.11.009. PMC 2575079. PMID 18555683.
- ^ Norman T. Adler; Donald Pfaff; Robert W. Goy (6 December 2012). Reproduction. Springer Science & Business Media. pp. 630–. ISBN 978-1-4684-4832-0.
- ^ Lo TE, Andal ZC, Lantion-Ang FL (2015). "Fluoxymesterone-induced gynaecomastia in a patient with childhood aplastic anaemia". BMJ Case Rep. 2015: bcr2014207474. doi:10.1136/bcr-2014-207474. PMC 4434366. PMID 25948845.
- ^ Kirschbaum J (27 October 1978). Profiles of Drug Substances, Excipients and Related Methodology. Academic Press. pp. 253–. ISBN 978-0-08-086102-9.
- ^ Mayer M, Rosen F (1975). "Interaction of anabolic steroids with glucocorticoid receptor sites in rat muscle cytosol". Am. J. Physiol. 229 (5): 1381–6. PMID 173192.
- ^ Gordan, G. S. (1976). "Cancer in Man": 499–513. doi:10.1007/978-3-642-66353-6_16.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 504–. ISBN 978-3-642-66353-6.
- ^ a b c d J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 568–. ISBN 978-1-4757-2085-3.
- ^ a b c d Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. p. 461. ISBN 978-3-88763-075-1.
- ^ Schänzer, Willi; Opfermann, Georg; Donike, Manfred (1992-11-01). "17-Epimerization of 17
α -methyl anabolic steroids in humans: metabolism and synthesis of 17α -hydroxy-17β -methyl steroids". Steroids. 57 (11): 537–550. doi:10.1016/0039-128X(92)90023-3. - ^ a b I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 123–. ISBN 978-94-011-4439-1.
- ^ a b c https://www.drugs.com/international/Fluoxymesterone.html
- ^ a b "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 17 December 2016.
- ^ Steven B. Karch, MD, FFFLM (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
{{cite book}}: CS1 maint: multiple names: authors list (link)
Further reading
- Daniels, R. C. (February 1, 2003). The Anabolic Steroid Handbook. Richard C Daniels. p. 80. ISBN 0-9548227-0-6.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help)
