Lactam

A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words lactone + amide.
Nomenclature
[edit]Greek prefixes in alphabetical order indicate ring size.
| Ring size (number of atoms in the ring) |
Systematic name | IUPAC name | Common name(s) | Structure |
|---|---|---|---|---|
| 3 | Aziridin-2-one |  | ||
| 4 | Azetidin-2-one | 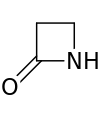 | ||
| 5 | Pyrrolidin-2-one |
|
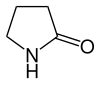 | |
| 6 | Piperidin-2-one |
|
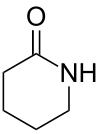 | |
| 7 | Azepan-2-one |
|
 |
This ring-size nomenclature stems from the fact that hydrolysis of an
Synthesis
[edit]General synthetic methods are used for the organic synthesis of lactams.
Beckmann rearrangement
[edit]Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
Schmidt reaction
[edit]Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms
Cyclization of amino acids
[edit]Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule.
Lactamization is most efficient in this way if the product is a
Intramolecular nucleophilic substitution
[edit]Lactams form from intramolecular attack of linear acyl derivatives from the nucleophilic abstraction reaction.
Iodolactamization
[edit]An iminium ion reacts with a halonium ion formed in situ by reaction of an alkene with iodine.[2]
Kinugasa reaction
[edit]Lactams form by copper-catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
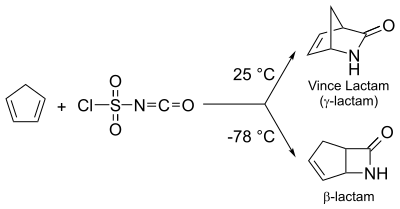
Diels-Alder reaction
[edit]Diels-Alder reaction between cyclopentadiene and chlorosulfonyl isocyanate (CSI) can be utilized to obtain both
Lactam–lactim tautomerism
[edit]A lactim is a cyclic imidic acid compound characterized by an endocyclic carbon-nitrogen double bond. They are formed when lactams undergo tautomerization.
Reactions
[edit]- Lactams can polymerize to polyamides.
See also
[edit]- Lactone, a cyclic ester.
β -Lactamβ -Lactam antibiotics, which includes penicillins- 2-Pyrrolidone
- 2-Piperidinone
- Caprolactam
References
[edit]- ^ Lam, Pak-Lun; Wu, Yue; Wong, Ka-Leung (30 March 2022). "Incorporation of Fmoc-Dab(Mtt)-OH during solid-phase peptide synthesis: a word of caution". Organic & Biomolecular Chemistry. 20 (13): 2601–2604. doi:10.1039/D2OB00070A. ISSN 1477-0539. PMID 35258068. S2CID 247175352.
- ^ Spencer Knapp, Frank S. Gibson Organic Syntheses, Coll. Vol. 9, p.516 (1998); Vol. 70, p.101 (1992) Online article
- ^ Singh, R.; Vince, R. Chem. Rev. 2012, 112 (8), pp 4642–4686."2-Azabicyclo[2.2.1]hept-5-en-3-one: Chemical Profile of a Versatile Synthetic Building Block and its Impact on the Development of Therapeutics"
- ^ Pham, P.-T.; Vince, R. Phosphorus, Sulphur and Silicon 2007, 779-791.
External links
[edit] Media related to Lactams at Wikimedia Commons
Media related to Lactams at Wikimedia Commons